|
Neurulation in a Salamander over a thirty year period. Boundaries between domains of cells are organizing sites for many events of neurulation. One boundary is between future neural plate and epidermis. Another boundary is between neural plate and notoplate (that part of the plate that overlies the notochord). Three conditions are invoked at the boundaries: (1) cells do not mix across the boundary, (2) cells that contact the boundary are trapped there by contact inhibition, yet adhere to the other cell type, (3) cells from both sides of the boundary intercalate with cells of their own kind at the boundary, are trapped at the boundary, and thus extend the length of the boundary. How early in development are these boundaries established? Zhang and Jacobson (1993) (Devel. Dyn. 196:79-90) found that the position of the anterior edge of the neural plate is fixed in the blastula of the Xenopus embryo before gastrulation commences at stage 9. There is no information for the salamander, but the timing is likely similar. Fate maps on the blastula define the areas that will later become neural plate, epidermis, mesoderm, and endoderm. Prospective neural plate (NE) occupies one quadrant of the surface of the spherical blastula; prospective epidermis (i.e. non-neural ectoderm, EE) occupies an adjacent quadrant of the surface of the spherical embryo . The rest of the spherical surface is occupied by future mesoderm (red) and endoderm (yellow). TM = tail mesoderm. DL = site of initiation of the dorsal lip. 
At this blastula stage (8+), both prospective neural plate (NE) and epidermal (EE) areas are several cells thick and overlie the blastula cavity, the blastocoel. Gastrulation starts at stages 9 and 10 as the dorsal lip (DL) of the blastopore forms, mesoderm rolls inward around the lip, and endoderm rolls inward beneath the lip. Lip formation extends laterally at stage 11, and by stage 12, the blastopore is completed ventrally to make the large yolk plug stage. 
Future neural plate and epidermis remain on the surface, and their domains are extended as the mesoderm and endoderm go inside the embryo. By stage 12, neural plate occupies the dorsal half of the surface, and the epidermis the ventral half. During gastrulation, the boundary between neural plate and epidermis of the blastula stage doubles in length, and each ectodermal domain has doubled its surface area. 
How do the domains of both neural plate and epidermis manage to double surface areas during gastrulation? At stage 12, both neural plate and epidermis are just one cell thick in salamander and amniote embryos. Between stages 8+ and 12, the near doubling of surface area in each of these domains was most likely a result of radial intercalation of deeper cells into the surface layer of cells. During gastrulation, the doubling of the length of the boundary between epidermal and neural plate domains seems to occur in a rather complex way. 
The endodermal mass rotates, turning inward beneath the blastopore lip, dragging the adjacent epidermal domain ventrally toward the posterior pole. At the same time, the prospective dorsal mesoderm converges toward the forming blastopore lip and involutes around the lip. The involuting dorsal mesoderm drags the dorsally positioned neural domain toward the top of the lip. Thus the neural and epidermal domains are stretched in opposite directions, pivoting at the point on their common line that separates them where it touches their boundary with the future tail mesoderm. The tail mesoderm converges towards the posterior midline as it thickens so its surface component thins and disappears, which closes and extends the boundary between epidermis and neural plate. The doubling of the length of the epidermal-neural boundary during gastrulation may thus be due mainly to the conversion of tail mesoderm-neural boundary into epidermis-neural plate boundary. The possibility of planar induction between tail mesoderm and elongating neural plate during gastrulation seems likely. What does the fate map of the neural plate look like at the end of gastrulation (stages 12-13)? 
The map above is from Jacobson and Gordon, 1976, their figure 23 (a pdf 5.8 MB is here ). The map was created by projecting the fate map of stage 15 of the axolotl onto a stage 15 newt, Taricha torosa, then choosing naturally pigmented cells and cell groups at or near and in brain part boundaries and following those pigmented points while running the time-lapse movie backwards to a stage between stages 12 and 13. The Keller group has made a similar remapping for Xenopus. (Keller, Shih, & Sater, Devel. Dyn. 13:199-217. 1992.) Many observations suggest forebrain is the default condition for neural plate. For example, only forebrain forms in lithium treated embryos. At the end of gastrulation, the re-mapped neural plate fate map (above) shows that prospective brain occupies nearly the entire neural plate. The part of the prospective neural plate that bordered the lateral wings of the future tail mesoderm during gastrulation is fated in this map to be mostly hindbrain. Could a planar induction have occurred between tail mesoderm and neural plate during gastrulation that diverted the contact area from default forebrain to hindbrain expression? In Xenopus, explants of prospective forebrain are induced to form hindbrain when combined with tail-end neural plate, and hindbrain combined with tail-end neural plate is induced to form spinal cord (G. Cox and A. Hemmati-Brevanlou, Development 121:4349-4358. 1995). In these experiments, tail-end neural plate includes tail mesoderm. Is the prospective tail mesoderm already determined as such at early gastrula stages? The answer is yes. Woodland and Jones found that tail mesoderm is induced as such by stage 10+ in Xenopus (Roux's Arch Dev Biol 19:441-446.1988). So by the beginning of gastrulation, tail mesoderm is in position to change the fate of adjacent neural plate by planar induction. Prospective notoplate cells initially compose the most posterior rim of the neural plate at the boundary between neural plate and tail mesoderm. Being a component of the neural plate domain, notoplate cells do not involute, but mimic the convergence-extension movements of the underlying notochordal cells. During gastrulation, notoplate cells gather at the midline of the posterior neural plate, having moved toward the dorsal lip of the blastopore by convergence. They then extend cranially in the midline position of the floor plate. The tail mesoderm, which also gathers at the dorsal lip is last to involute, and is left behind at the dorsal lip as part of the tailbud. Each cell of an amphibian embryo contains a supply of yolk platelets, so growth is not possible until the heart first beats at stage 34 and blood can carry nutrients from the large endodermal cells. There is no growth in the prospective nervous system during neurulation. Studying neurulation in amphibian embryos is not confused by growth, a distinct advantage. Bird and mammal embryos do grow, actually considerably, during neurulation. Far from being one of the mechanisms of neurulation in these taxa, growth rather hinders neurulation. Far more neural birth defects are found in birds and mammals than in amphibians. In forty to fifty years of looking at amphibian embryos, I have never seen a case of natural spina bifida, but in chick embryos, during a hot Texas summer, I have seen neural tube defects in up to half of the embryos. Gastrulation is concluding as neurulation begins. Between stages 12 and 14, the neural plate is forming. The embryo is a sphere at stages 12 and 13 and the prospective neural plate occupies the dorsal surface. By stage 14, the neural plate has changed from a hemisphere to (approximately) a disc. The forming neural plate flattens and thickens, though it remains just one cell deep. 
Simple geometry says conversion of a hemispheric shell to a disc of the same diameter reduces the surface area by half and doubles the thickness of the hemispherical shell. Our measurements of embryos between stages 12 and 14 found that the cell heights of the neural plate cells had doubled as the surface area halved (Jacobson and Gordon, 1976, p. 210). The apical surfaces of the plate cells compose the plate surface. Each cell is cylinder-shaped at stage 14. Each apical surface reduced by half between stages 12-13 and 14, and did so, no doubt, by contraction of the subsurface purse string of microfilaments as each cell elongated. Cell elongation also results in reduction of apical cell surface. Contraction of the purse string of subapical microfilaments would reduce apical surface, but could turn the cell into many shapes besides a cylinder. How the cells remain cylinders requires further explanation. The flat neural plate at stages 13+ to 14 is fairly uniformly thick. The plate cells all get taller to double plate thickness by stage 14. The environment of the basal surface of each cell changes drastically between stages 12 and 14. Migrating head endoderm and mesoderm drag themselves along against the plate bottom followed by involution and convergence and extension of the mesoderm that will be notochord and somites. Extracellular matrix between mesoderm and neural plate bottom is also most likely changing. Elongation of the cells of the neural plate could be affected by these changes at the basal surfaces. 
The SEM's above, all at 1500 micrometers, are of the apical surfaces of neural plates in the hind brain region at (A) stage 13, (B) stage 15, (C) stage 19. From 'Morphogenesis of the Neural Plate', Fig 10, by A.G. Jacobson, in 'Morphogenesis and Patterm Formation', T.G. Connelly et al., eds. Raven Press, New York, 1981. Apical surface areas of the plate cells decrease. 
SEMs above of (A) edge and underside of newt neural plate at stage 13 (500X). Mesoderm has separated cleanly from the underside. (B) Stage 13 neural plate basal surfaces (1500X). Numerous filopodia (small arrows) stretch across the basal surfaces and between cells. Some broad lamellipodia (arrow heads) pass from cell to cell. From: A.G. Jacobson in Connelly et al., his fig 18. The plate cells are roughly cylinders. There is no evidence that apical constriction caused wedging or elongation of the cells. To the contrary, it appears that elongation of the cells dominates, as shown in the next sem. These scanning electron micrographs show no hint of ECM at the basal surfaces. 
The SEM above (fig. 19 from the reference above) shows the stage 13 neural plate edge at 700X. 'Wedging', where seen, is in the direction opposite what would be expected if caused by contraction of subapical microfilaments. Lateral lamellipodia are very evident. What does looking at the details of the stage 13 neural plate tell us about how the plate cells double their cell heights between stages 12 and 14? While reduction of apical surfaces accompanies increase in cell height, subapical contriction does not appear to be the driving force. Rather, whatever the cell elongation mechanism or mechanisms are, they seem to lead. If cortical tractoring is doing the elongation, and I suspect it is, then there must be some sort of differences in tractoring rates so elongating cells can tractor on their neighbors. Differences among the neighbors are apparent, and lamellipodia stretching between cells suggest cells may be changing neighbors. Views of the apical surfaces of the plate cells indicate that few if any of the apical cell surfaces are in stable hexagonal Weliky-Oster arrays. They are likely changing neighbors frequently at these early stages. This problem of 'placoding'is taken up later when we visit finite element models. The notoplate cells of the neural plate have converged and gathered toward the midline and bulge craniad into the prospective brain area by stage 13. Jacobson and Gordon (1976, page 216) observed that the notoplate region does not cleave through the plate, but rather displaces cells craniad. The illustration below shows this. Where the diagram label is "notochord", it should be "notoplate". I counted the number of brain cells on a line between the anterior tip of the notoplate and the anterior edge of the neural plate at stages 13 and 15 (Jacobson and Gordon, 1976, p.217) (pdf.5.8 MB is here ). At both stages there were 80 cells.  Like the notochord that underlies the notoplate, the most anterior end of the notoplate lies in the midline of the midbrain. It appears to do so already at stage 13. This is shown in the next two illustrations. The illustration below of a stage 15 newt embryo has a notoplate with very little pigment in it, so the notoplate appears as a white streak from the midbrain posteriorly down the midline through the hindbrain and spinal cord. The notoplate position at stage 13 was reported in Jacobson and Gordon (1976, page 211). In the illustration following, I have superimposed the notoplate region at stage 13 on the remapped neural plate to show how the anterior-most notoplate cells are already at the midbrain level at this stage. These illustrations also help make clear that neural plate elongation between stages 13 and 15 is primarily in the spinal cord region whose cells mostly emerge from the ventral sides of the embryo. The cells of the brain accommodate the lengthening notoplate area mostly by condensing at the midline by becoming taller in the brain region anterior to the notoplate. 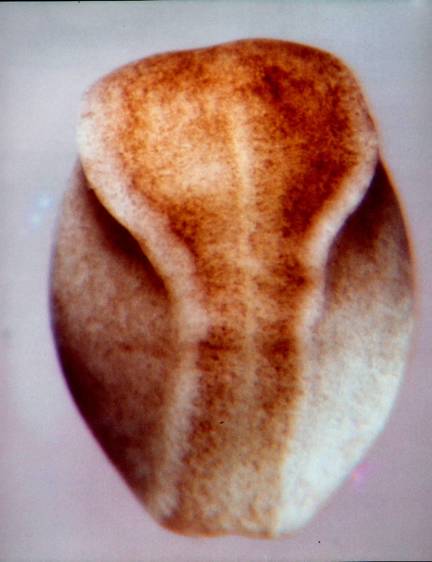

Burnside used a time-lapse movie to follow cells at the intersections of a superimposed coordinate grid from her stage 13 to stage 15. This allowed her to define cell trajectories between these stages, and simultaneously to determine changes in area on the grid. Below is an illustration of her starting grid on her stage 13 embryo to which I have added an outline of the notoplate area. 
Below are the cell trajectories from Burnside and Jacobson, 1969, page 541. 
Cells at the midline (not shown) moved craniad along the midline, or at the caudal end, caudad along the midline. The cell trajectories on the left half were mirror images so are not shown. The trajectories are quite complex. The cells from B to G moved mostly toward the midline and craniad. Cells starting at A instead moved toward the midline and caudad. At the midline between A and B is the point of no cell movement, called the 'dead point' by Jacobson and Gordon, 1976. It is fortunate that Burnside used a somewhat older stage 13 or this set of differences would have been missed. Burnside's cell trajectories are essential to understand the biomechanics of neurulation. Between stages 13 and 15, the neural plate elongates considerably. Since at stage 15 the plate is wrapped around the dorsal hemisphere and into the ventral hemisphere, optical foreshortening does not make the plate elongation obvious. To measure plate lengths without the optical foreshortening, I excised the plates, placed them on a bed of agar, and measured them immediately. (See illustration below.) An instantaneous relaxation of apparent elastic mediolateral tensions of the stage 15 plate occurs at excision from the epidermis. The plate snaps to a narrower configuration. Apical surfaces of spinal cord cells at stage 15 are stretched in the mediolateral direction sometimes to as much as ten times their anterior-posterior width (Jacobson and Gordon, 1976). 
Jacobson and Gordon (1976) used the Burnside cell trajectories and area changes for their computer simulation of shape changes in the neural plate between stages 13 and 15. They invoked the elongation just described, and a pattern of shrinkage as the driving forces for the changes of plate shape. The shrinkage is due to reduction of apical surfaces of cells as the cells get taller. We established that there is a programmed pattern of shrinkage on the plate surface in several ways. We excised a stage 13 neural plate with no other tissues. A pigment pattern of taller cells was imprinted on the surface when control embryos reached stage 15 (below). (From Jacobson and Gordon, (1976) p.217). 
At stage 13, we also transplanted pieces of prospective high shrinkage to areas of low shrinkage, and vice versa, and the transplants got taller by their programmed amount of shrinkage despite the foreign environments. Interestingly, an implant of epidermis into spinal cord neural plate continues to spread while the neural plate cells around the implant continue to reduce their surface areas and get taller. Below is a summary of the Jacobson and Gordon (1976) computer simulation. 
The Jacobson-Gordon simulation helped understand the long period when the neural plate converts its shape from a hemisphere to a flat keyhole shape at stage 15. How the neural plate rolls into a tube in the much shorter period between stages 15 and 20 was not addressed. About ten years after the Jacobson-Gordon simulation, George Oster, Garry Odell, and Louis Cheng, and I developed a model of cell behavior in the folding neural plate and did finite element simulations to test the model. This work appeared as 'The Cortical Tractor Model for Epithelial Folding: Application to the Neural Plate', by AG Jacobson, GM Odell and GF Oster, in 'Molecular Determinants of Animal Form', pp. 143-166, GM Edelman, ed., 1985, Alan R. Liss, Inc., New York., and 'Neurulation and the cortical tractor model for epithelial folding', AG Jacobson, GF Oster, GM Odell, and LY Cheng, JEEM 96:19-49, 1986. In this model, the nature of how cells crawl (GF Oster, (1984) On the Crawling of Cells. JEEM 83 (Suppl., 329-364.)) is extended to cells that change neighbors in intact epithelia. The basics of the cortical tractoring model are accepted by many for crawling of mesenchymal cells. The cortical cytoplasm, with its actomyosin contractile components flows from the site of cell protrusions toward the trailing end of the cell, carrying with it attached adhesive molecules that penetrate the cell membrane and attach directly or indirectly to the substrate or to other cells. The cortical flow carries along the adhesive molecules, and contractions of the cortical cytoplasm may move the cell. At the sink end of the cell, the cortical cytoplasm fountains into the cell interior and the adhesion molecules are absorbed into the cells as well. We find that the neural plate (and other epithelia) have the same mechanisms, but epithelial cells remain bound one to another at the apical ends of the cells. Epithelial cells can and do nevertheless move about within the plane of the epithelial sheet, while preserving the apical seals that protect them from the environment. 
Schematic of a cross section of an epithelial sheet. The time-averaged flow of the cortex in each cell comprises the cortical tractor motion (arrows). The cortical flow inserts adhesive structures at the basal source, and reabsorbs them in the apical region. These junctional molecules pile up at the apical end and form the apical seal, a structure that is constantly being renewed. As long as the time-averaged speed of the cortical tractor in adjacent cells remains approximately equal, no shear forces will be generated, and no deformation of the epithelial sheet will take place, (From Jacobson, Oster, Odell, and Cheng, 1986) Epithelial cells do not normally make protrusions from their apical ends, but lamellipodia often protrude from lateral surfaces, and from basal surfaces. The cortical tractor model proposes that there are successive basal protrusions in which the actin network solubilizes, followed by rapid re-polymerization initiating a wave of contraction toward the apical end of the cell. Holtfreter illustrated just such waves of contraction in isolated amphibian neural plate cells (J.Holtfreter, Structure, Motility and Locomotion in Isolated Embryonic Amphibian Cells, J. Morph. 79:27-61, 1946). Holtfreter illustrated an isolated cell with the basal end up and the apical end down. 
The tractoring of neural plate cells on the epidermis at the neural plate-epidermal boundary is the best explanation of the raising of a neural fold and the rolling of the fold toward the midline of the plate, ending in the closure of the plate into a tube. Many observations and experiments support this assertion. Some of them follow this statement of how tractoring between neural plate cells and epidermis raise the neural folds and then close the folds into a tube. The cells of the neural plate abut cells of the epidermis at the neural plate-epidermis boundary. The two cell types will not mix, and abutting cells are stuck at the boundary by greater adhesion between them, and by contact inhibition. A neural plate cell at the boundary tractors against epidermal cells, elongating the plate cell. The next more-medial plate cell now tractors against the elongating cell and contacts epidermis at its basal end to tractor against the epidermis, and a wave of contraction moves from the basal end of the cell to the apical end, producing a rolling moment, lifting the epidermis up over the neural plate. This process progresses incrementally mediad. The result is a rising of the epidermis up over the edge of the neural plate producing a neural fold. This rolling moment of the epidermis is quite visible and is not explained by other models. 

This section of a T. torosa neurula shows tractoring and rolling as in the bottom right of the diagram above the sections. 
In sections such as this one, the notoplate consists of those cells in the plate whose basal ends contact the underlying notochord. Neural plate cells lie between two boundaries, the epidermal boundaries at the lateral edges, and the notoplate boundaries at the midline. 
The above sections are 'plastic thin sections', each 2 microns thick. The section directly above is through the posterior hindbrain. The neural tube is about to close. The neural folds contain many loose neural plate cells that will be future neural crest cells. The epidermis from each side has been drawn deep into the neural groove. When the folds on each side meet and fuse at the top of the groove, many epiderml cells are trapped in the groove and are also likely future neural crest cells. As notoplate cells rearrange by cortical tractoring, some contact the boundary and are stuck there. During neurulation, the notoplate becomes increasingly more narrow and longer in the anteroposterior axis. Experiments show that cutting along the notoplate-neural plate boundary essentially stops midline elongation. Plate elongation during neural tube closure (between stages 15 and 19) goes about ten times faster than before or after neurulation stages in T. torosa. In the chick embryo, the closing portion of the neural tube also elongates much more rapidly than closed or open regions. These rate differences are shown below in illustrations from A.G. Jacobson, 1981, Morphogenesis of the Neural Plate and Tube, in Morphogenesis and Pattern Formation, T.G. Connelly et al. eds., Raven Press, New York. 


We measured changing lengths of neural plate-epidermal boundary in newt and axolotl embryos during neurulation (AG Jacobson and JD Moury, Tissue boundaries and cell behavior during neurulation, Devel. Biol. 171:98-110, 1995.). Neural folds at the edges of the brain plate decrease in length during neurulation, while neural folds abutting the spinal cord plate increase in length during neurulation. 
We explanted axolotl neural plates with underlying tissues and a rim of adjacent neural fold epidermis (A) or without adjacent neural fold epidermis (C). The photographs below were made within one minute of the operation. When intact axolotl control embryos reached closed-tube stage 20-21, the same explants were photographed in dorsal view. (B) the explant with epidermis has closed into a tube. (D) the explant without epidermis remains an open plate except in the most caudal spinal cord. Bar = 1mm. 
These observations and experiments strongly suggest that continued interaction between neural plate and epidermis is essential for closure of the neural tube. In another experiment we explanted a stage 15 neural plate with epidermis removed from on side and not from the other. Only the side with epidermis rolled, the side with no epidermis remained flat. This is illustrated below (From Jacobson and Moury, 995). 

In the newt, many cells, especially in the hindbrain region, detach from the neuroepithelium and are stored within the edges of the neural plate, that is in the neural folds. They are future neural crest cells. At the same time, epidermis is being rolled into the valley between the folds, and they are likely future neural crest cells as well. In bird and mammal embryos, neural crest cells are shed from the neural folds while the plate is rolling into a tube, especially in the hindbrain region of the plate. The folds do not elongate in brain regions of the plate most likely because fold cells are being stored loose or shed rather than intercalating at the boundary and contributing to elongation of the folds. Also, the cells of the brain plate are those that get tallest, so without much intercalation at the boundary, the folds should get shorter. The expansive brain plate offers more problems of tube formation than the narrower spinal cord region. The mouse brain plate shown below (from Jacobson and Tam, 1982) shows both the problems of large-brained organisms, and the power of the neural plate-epidermis boundary to roll the plate into a tube. 
The cortical tractor model seems best to provide the necessary forces to initially thicken the neural plate, to form neural folds and to lengthen or shorten the folds, and later to form the plate into a tube. When David Moury was starting as a doctoral student, I suggested to him that if the cortical tractor model was correct, then any piece of neural plate apposed to any piece of epidermis should form a neural fold between them. Moury found that to be quite true. To distinguish donor from host tissues, he used albino embryos (with no egg pigment) and black normal embryos (J.D.Moury and A.G. Jacobson, Neural fold formation at newly created boundaries between neural plate and epidermis in the axolotl, Devel. Biol. 133:44-57 (1989; Moury and Jacobson. The origins of neural crest cells in the axolotl, Devel. Biol. 141:243-253 (1990)). A piece of neural plate implanted into the ventral epidermis resulted in a fold forming around the entire implant. The new neural folds around the piece of implanted neural plate shorten and the implant forms into a neural vesicle. Neural crest cells are produced in the shortening neural folds. A piece of epidermis implanted into the neural plate formed neural fold around the entire implant. Neural folds at the edge of the epidermal implant also formed neural crest cells. These experiments clearly show that both epidermis and neural plate produce neural crest cells. Strangely, neural plate made mostly melanocytes, and epidermis produced spinal and cranial ganglion cells. Reciprocal induction between neural plate and epidermis is largely responsible for elicitation of neural crest cells. Some ideas that emerge from the material covered so far: There are three sorts of neurulation in the amphibisn embryo. The brain has one set of rules, the hind-brain spinal cord another set, and the tail a third set of rules. The spinal cord in the tail emerges from (probably sorts out in) the tail bud. Others have described this long ago, and it is called 'secondary neurulation'. The posterior sixth of the 'neural plate' was recognized long ago as containing the tail somites as well as neural tissue. In the Burnside mapping of cell trajectories, there was a motionless dead point, rostral to which plate cells move craniad, and posterior to which plate cells move caudad. The latter cells form the tail bud. Tail bud formation may hark back to common ancestors of the chordates. Vertebrate embryos have a head, a trunk, and a tail. The fate maps of the neural plate in salamanders and Xenopus, when projected back to closed blastopore stages, show that most of the forming neural plate is future brain. Trunk and tail parts are still emerging from being wrapped ventrally around the blastopore. I have found an illustration that may show the extent of the ventral placement of trunk and tail plate in the newt. This SEM of a stage 12+ Taricha torosa embryo is a dorsal-posterior view of the neural plate. Along the left side, where the 'lighting' best shows it, is seen the line between neural plate and epidermis deflecting down ventrally to the ventral lip of the blastopore. 
Compare the map below with the SEM above. 
In these dorsal views, many of the hindbrain cells and most of the spinal cord cells are located ventral to the edges of those parts as seen from above. Most of the cells in the neural plate of the closing blastopore stage are cells of the brain. The Jacobson-Gordon shrinkage map indicates that apical surfaces of the forebrain cells shrink the most. These cells are also displaced in a wheeling or fountain movement. Cells at the forebrain midline move mostly rostrally. Wheeling movements in the forebrain displace rostral cells laterally, and more lateral cells caudally along the neural plate-epidermis boundary. The wheeling movements of diencephalic forebrain cells in the neural plate are especially well understood for the rudiments of the eye. The eye rudiments begin as a single unit in the midline of the diencephalic neural plate. This single rudiment depends on the presence of underlying prechordal plate to split and begin the journey to final lateral positions. Adelmann showed long ago that removal of the prechordal plate resulted in cyclopic embryos. R.B. Brun has traced the pathways of the eye rudiments in axolotl and Xenopus embryos (RB Brun, The movement of the prospective eye vesicles from the neural plate into the neural fold in Ambystoma mexicanum and Xenopus laevis. Devel. Biol. 88:192-199.). The single rudiment splits in two then the trajectories of the two rudiments are right and left arcs anteriad, then laterad, then posteriad. That is, a fountain or wheeling movement. Other cells of the forebrain make similar journeys. The mediolateral divisions of the neural plate (floor plate, basal plate, alar plate, and roof plate) are found in the spinal cord, hindbrain, and midbrain, but not in the forebrain. Their extent anteriorly coincides with the anterior extent of the notoplate, which is in the floor plate position. The forebrain consists mostly just of alar plate. Several sources make clear that most of the telencephalic portion of the forebrain arises from the anterior neural ridge. This is shown in the map of the neural plate of Xenopus made by Eagleson and Harris (GW Eagleson and WA Harris, Mapping of the presumptive brain regions in the neural plate of Xenopus laevis, J. Neurobiology 21:427-440. 1990). 
In this Eagleson and Harris map of the Xenopus neural plate, '19' is the cerebellum. Continued wheeling after this stage moves the retina caudad along the lateral neural fold, implying that lateral mesencephalon regions also moved posteriad. The wheeling movements of the forebrain cells in plate stages likely moved forebrain parts to the lateral edges of the midbrain to form structures such as the cerebellum. These lateral reaches, of course, are dorsal in the closed tube. Starck has for years held the view that midbrain is composed of forebrain dorsally and hindbrain ventrally (D Starck (1975), 'Embryologie Ein Lehrbuch auf allgemein biologischer Grundlage (3.Auflage).' Stuttgart:Georg Thierne Verlag.) The neural tube thus has three rather different parts. A forebrain that is mainly alar plate, a hindbrain-spinal cord portion with floor, basal, alar, and roof plates, and a sort of hybrid between the forebrain and hindbrain, the midbrain, which is forebrain-like dorsally and hindbrain-like ventrally. The part on the map above labeled 23 is the future choroid plexus. Summary of studies of amphibian neurulation The boundaries between neural plate and epidermis, and between neural plate and notoplate have major roles in guiding the changing of shape of the neural plate. The anterior boundary between future neural plate and epidermis is established before gastrulation commences in Xenopus. During gastrulation, the area of the prospective neural plate doubles, as does the length of the neural plate-epidermis boundary. By the end of gastrulation, the prospective neural plate is a hemisphere of neural epithelium just one cell thick. A fate map of the neural plate re-mapped back to the end of gastrulation shows most of the area of the future neural plate hemisphere is future brain, and the future cells of the notoplate are already emplaced at the midline from the midbrain to the rim of the blastopore. The neural plate is established as cells of the neural hemispheric shell, while preserving volume, reduce apical surfaces by half and double cell height. This establishes a flat disc of neural plate with the same diameter as the original neural hemisphere. Cheng and Oster modeling shows that this sort of placoding may occur by cortical tractoring. During ensuing neurulation, the neural plate doubles its length, triples its thickness, reduces width ten-fold, greatly reduces apical surface area, and rolls into a tube. There is no growth during neurulation stages in amphibian embryos. The changes in shape of the neural plate are due to changes in shape and positions of the constituent cells of the neural epithelium. The dominant mechanism for increase in length of the neural plate midline is intercalation of notoplate cells and neural plate cells along the notoplate-neural plate boundary. In the spinal cord and hindbrain regions, intercalation of neural plate and epidermal cells along the neural plate-epidermal boundary also lengthens that boundary in concert with the midline elongation. The notoplate cells, during normal neurulation, are bound to the underlying notochord cells. The neural plate cells, lying between two elongating boundaries, are stretched perpendicular to the long axis. Apical surfaces of these cells are deformed so they are up to ten times as long in the mediolateral axis as in the craniocaudal axis. Being anchored at the midline and stretched mediolaterally may stiffen the plate and help keep it from wobbling while being stretched in the craniocaudal axis. The tripling of neural plate thickness during neurulation is the result of the constituent cells of the neural plate getting taller. As these cells get taller, their apical surfaces get smaller. It appears that most increase in cell height is due to cortical tractoring, but contraction of the subapical circumferential microfilament bundle could also help. Plate cells at the boundary with epidermis crawl beneath the epidermis, thus elongating themselves while lifting the epidermis in a rolling moment that forms the neural folds and moves the epidermal edge toward the midline. This process perpetuates among the plate cells toward the midine. For much of neurulation, the plate cells next to the notoplate border also increase their heights, and this effect declines laterally. The powerful rolling moment is the dominant force of tube formation, but the Poisson buckling that results from plate elongation could contribute some bending force as well. The ten-fold reduction of neural plate width during neurulation occurs mainly in the spinal cord-hindbrain regions where cells intercalate along plate boundaries increasing plate length at the expense of plate width. Some reduction of plate width is due to increase in cell heighths with concomitant decrease in apical surfaces and cell diameters. In the brain region, where the plate cells get especially taller. the reduction in surface area of the plate is accomplished mainly by decrease in the apical surfaces of the cells (see shrinkage pattern of Jacobson and Gordon, 1976). The semi-ring of plate boundary of brain plate with epidermis has some intercalation of cells at the boundary, but many boundary cells are pulled out of the epithelium and are stored in the neural folds as future neural crest cells, especially in the midbrain and hindbrain regions. Cells of the brain plate get especially taller, so each cell decreases its diameter during neurulation. Mostly, the brain neural plate-epidermal boundary gets shorter during neurulation rather than longer. The prosencephalon makes few, if any, neural crest cells. The mesencephalon, lying over the anterior tip of the notoplate, makes the largest contribution to the neural crest population, and the hindbrain and spinal cord also make large numbers of neural crest cells. The neural folds first contact one another and initiate fusion of the neural folds where the shortening brain plate boundary joins the elongating boundary of the hindbrain-spinal cord. 
While neurulation ends at stage 20, some of the neurulation processes continue. This is especially true of the role played by the notoplate in elongation of the nervous system. Neural tube elongation continues for some time after tube closure. The notoplate is several cells wide during neurulation. At the end of neurulation it is still four to six cells wide. These cells continue to intercalate at the notoplate-neural plate boundaries after tube closure, elongating the midline of the neural tube. In the closed neural tube, the notoplate cells are in the position of the floor plate, connecting laterally with the basal plates of the tube. Between stages 23 and 26, depending on the position along the craniocaudal axis, the notoplate becomes reduced to one cell wide, that cell being stretched in the mediolateral axis and tying together the two basal plates of the tube in which can be seen a new population of cells that will contribute to the floor plate. End of neurulation. |