|
Formation of the Amnion in the Chick Embryo The amniotes (reptiles, birds, and mammals) have four extraembryonic membranes, namely, the amnion, chorion, yolk sac, and allantois. Amnion and chorion are composed from extraembryonic somatopleure, which consists of ectoderm and somatic lateral plate mesoderm. Yolk sac and allantois are composed from extraembryonic endoderm and splanchnic lateral plate mesoderm. The amnion becomes a fluid-filled sac surrounding the embryo. The amniotic ectoderm faces the embryo and is non-adhesive with the ectoderm of the embryo. The somatic mesoderm of the amnion remains non-vascular, but forms some muscle which may contract in a slow rhythm "rocking" the embryo. This sac is thought to protect the embryo, and its arrangement of tissue prevents adhesions. The chorion and amnion form at the same time from the somatopleure by a fold that lifts the amnion up over the head and tail of the embryo. The chorionic ectoderm faces toward the shell of the egg where it eventually contacts the shell membranes, but does not adhere to them. Between the amnion and chorion is a cavity, the extraembryonic coelom. The yolk sac endoderm lies against the mass of the yolk. As the blastoderm disc expands, the cells at the leading edge of the yolk sac endoderm attach to the overlying vitelline membrane and are part of the mechanism dragging the yolk sac on its mission to surround the yolk mass. When extraembryonic splanchnic mesoderm forms, it inposes itself between the yolk sac endoderm and the vitelline membrane. The first vascular cells and blood arise in yolk sac mesoderm. The allantois is last to form and is an out-pouching of the hind gut. It expands rapidly into the cavity between the amnion and the chorion. The allantoic splanchnic mesoderm faces the somatic mesoderm of the chorion, and the two mesoderms fuse forming a composite membrane, the chorio-allantoic membrane, which is pushed against the shell membranes. This composite membrane serves as a respiratory organ, receiving oxygen through the pores of the shell and its membranes, and carries this gas to the embryo through the vascular system that develops in the splanchnic mesoderm of the chorio-allantoic membrane. Carbon dioxide passes out from the embryo by the same route. Waste products from the kidneys and bowel of the embryo are stored in the cavity of the chorio-allantoic membrane. The illustration below shows the disposition of these extraembryonic membranes in a chick embryo. Figure 1. 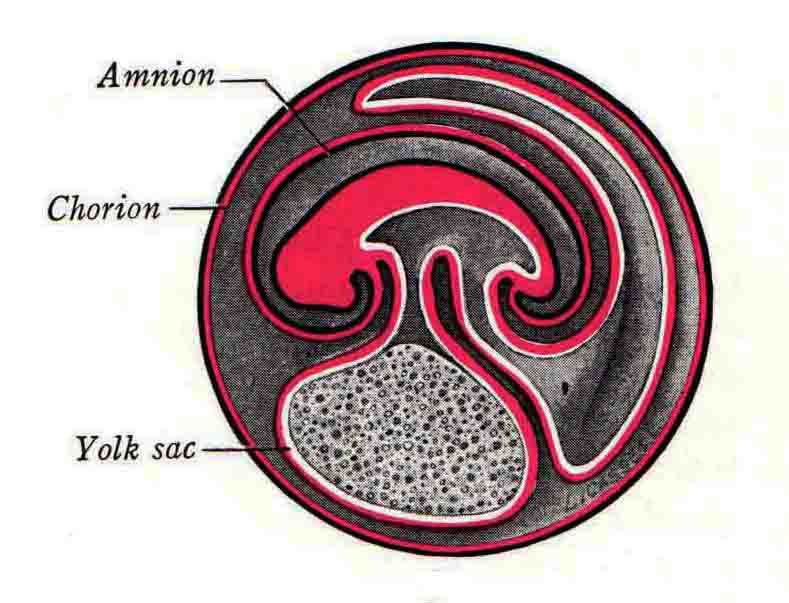 This diagramatic stereogram is a sagittal hemisection from Arey's Develpmental Anatomy textbook, seventh edition, revised. Ectoderm, black; mesoderm, red; endoderm white. The allantois bulges from the hind gut to the right. Forbes Davidson's fine doctoral research was a study of formation of the amnion. (Forbes Davidson, "A descriptive and experimental study of amniotic fold formation in the chick embryo." Ph.D. Dissertation, The University of Texas at Austin, Dec. 1977. Unpublished.) In the following account, the dissertation is shortened, sometimes paraphrased, and the order of presentation modified. [I (AGJ) sometimes insert comments (in brackets like this sentence) or make suggestions based on more recent works, or give my own interpretations.] Amnion formation begins at stage 10- (9 somites, 33 hours of incubation, and is generally completed by stage 18 (72 hours of incubation). The process therefore requires about 40 hours to complete at 38 degrees C. Stages are those of Hamburger, V. and Hamilton, H.L. (1951). J. Morph. 88:49-92. Davidson divided the formation of the amnion into three phases shown in his diagram below: Figure 2 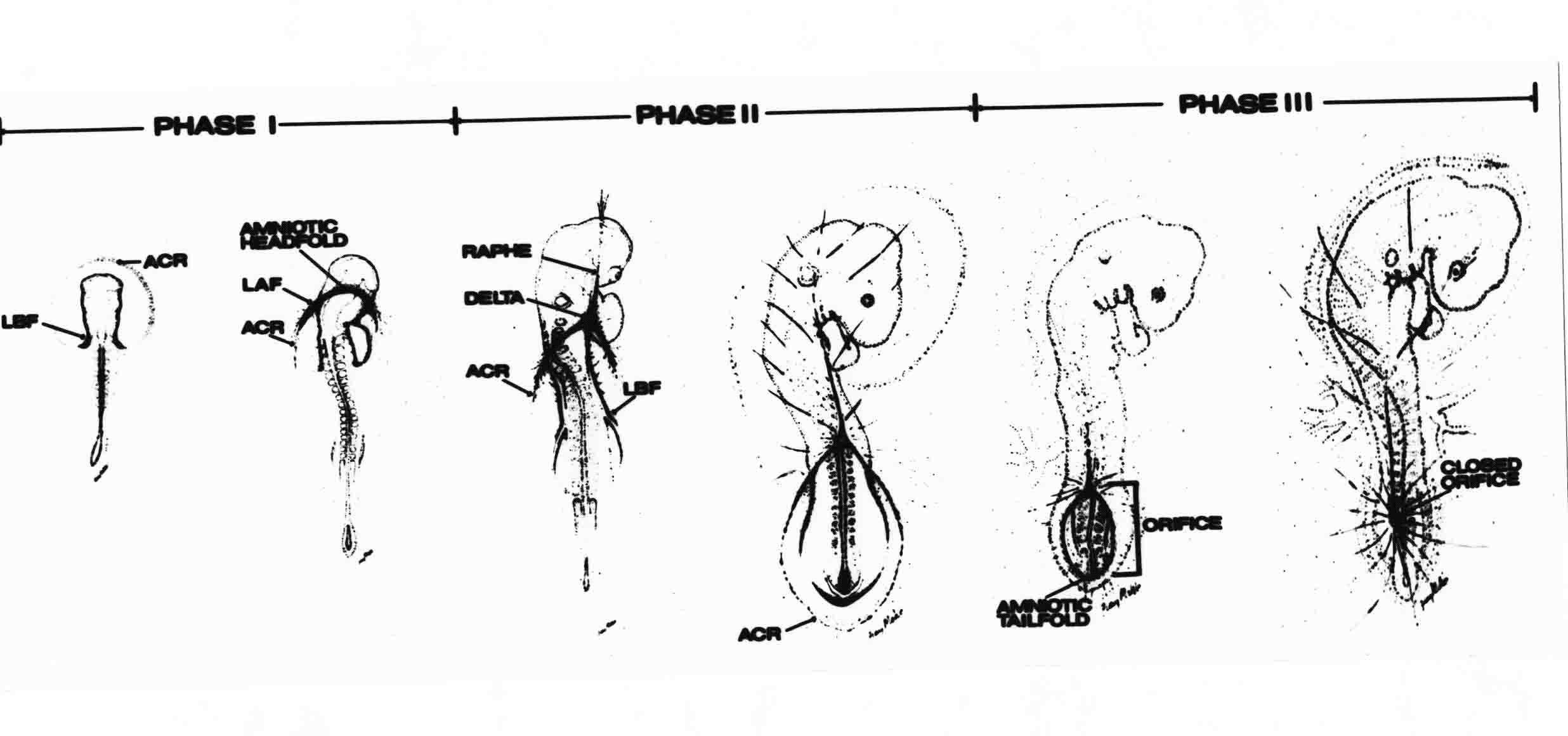 Phase 1 begins at Hamburger-Hamilton stage 10- with formation of an amniochorionic ridge (ACR) in the extraembryonic ectoderm around the front of the head. The caudal extension of the ACR coincides with the level of the lateral body folds (LBF). The ridge is a population of ectodermal cells, elongated along the ridge. The ACR becomes the amniotic fold, which rises and envelopes the head. The lateral amniotic folds extend caudad, and new ACR arises ahead of them. Figure3 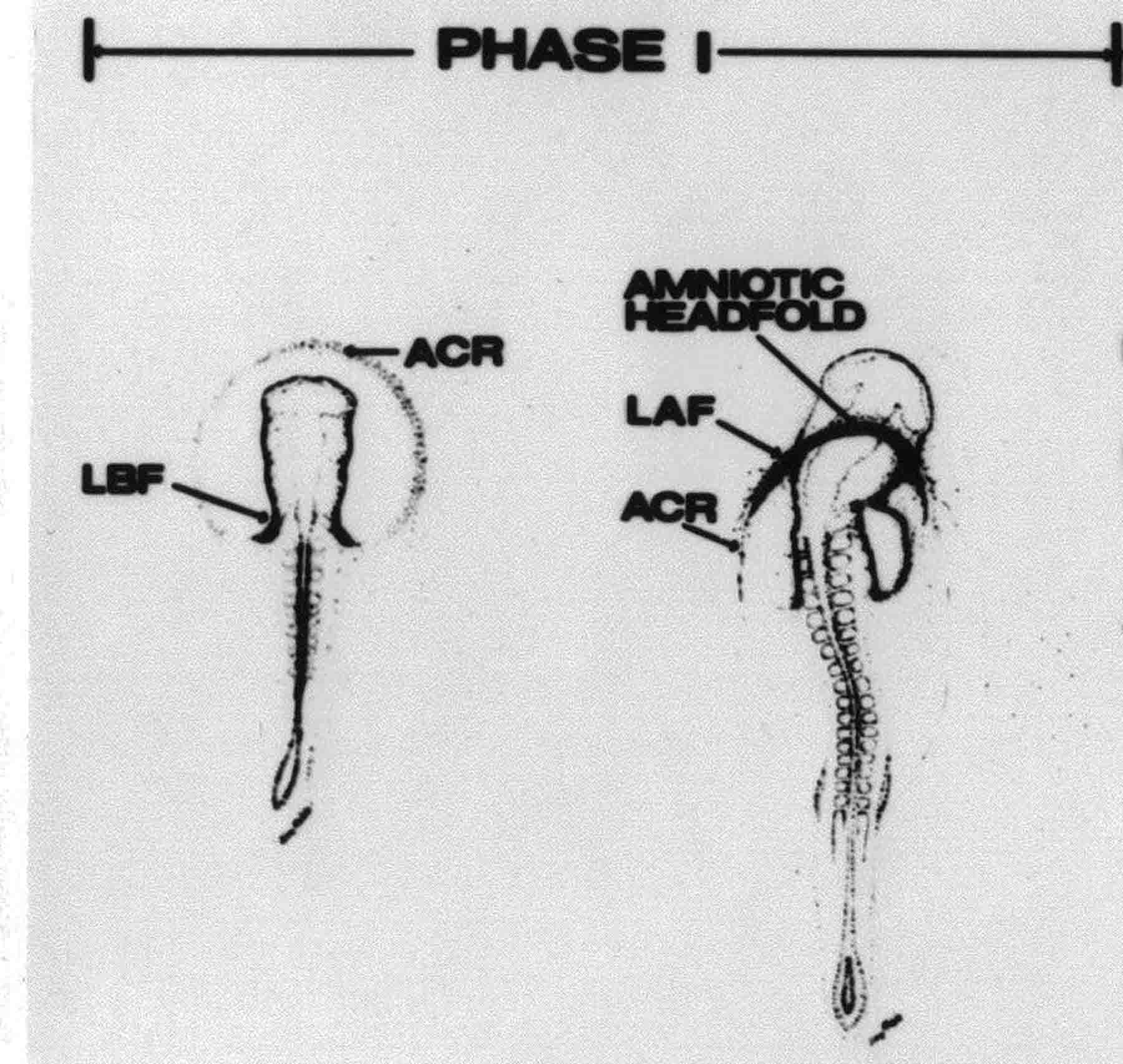 Phase ll begins about stage 13+. During Phase ll, an amniotic delta (DELTA) forms at the apex of the midline of the amniotic headfold. The seroamniotic raphe (RAPHE) extends rostrally from the delta. A nipple of cells may extend from the caudal face of the delta. The amniochorionic ridges and the body folds extend caudally simultaneously. Phase ll ends at about stage 17 once the ACR has extended behind and around the tailbud. Figure 4 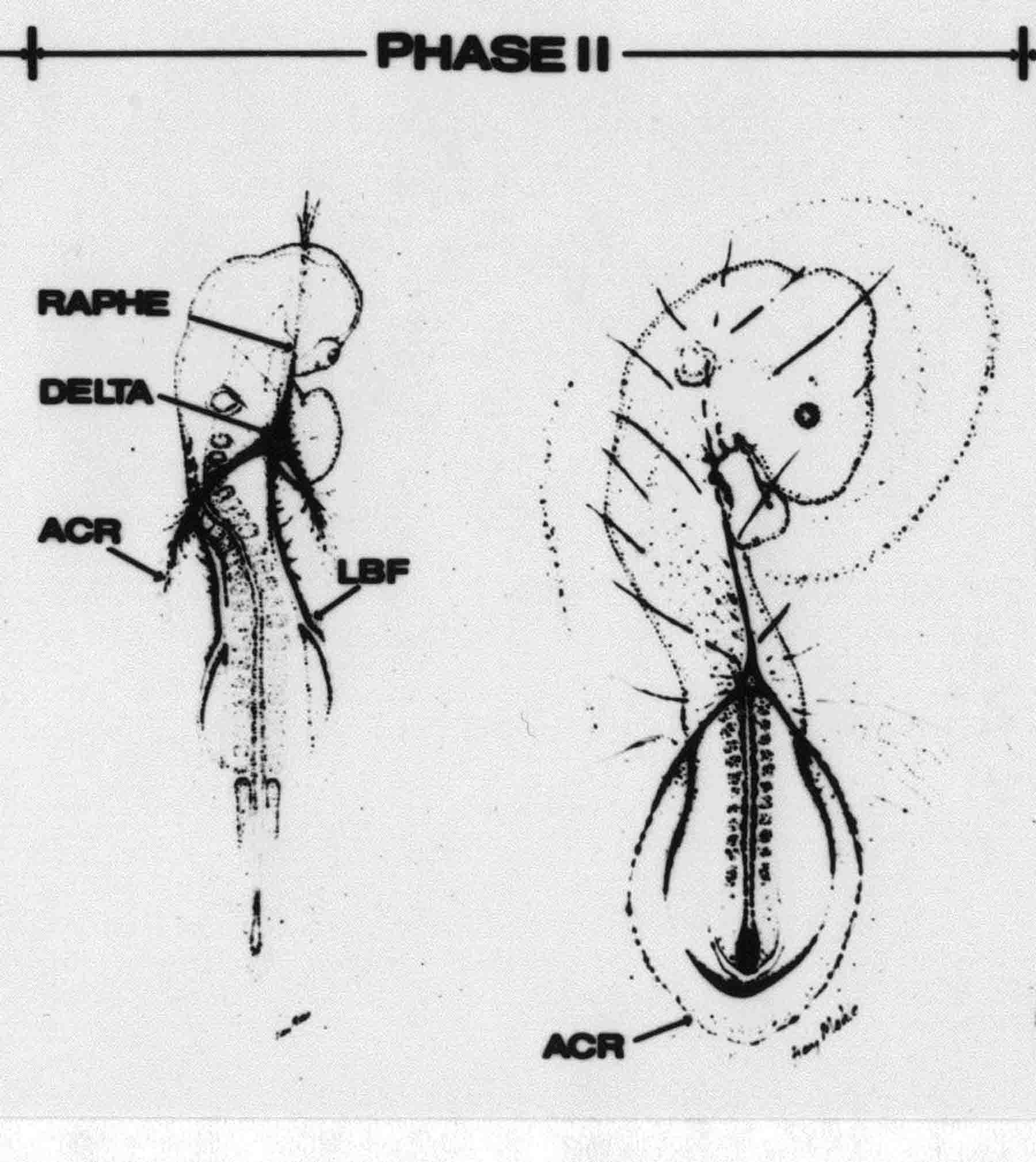 During Phase lll, the ACR around the tail fold converts to tail amniotic fold. Lateral ACR converts to lateral amniotic folds of each side which unite to form an orifice (ORIFICE), the amniotic orifice. This orifice then progressively becomes smaller until it closes off by stage 18. Figure 5 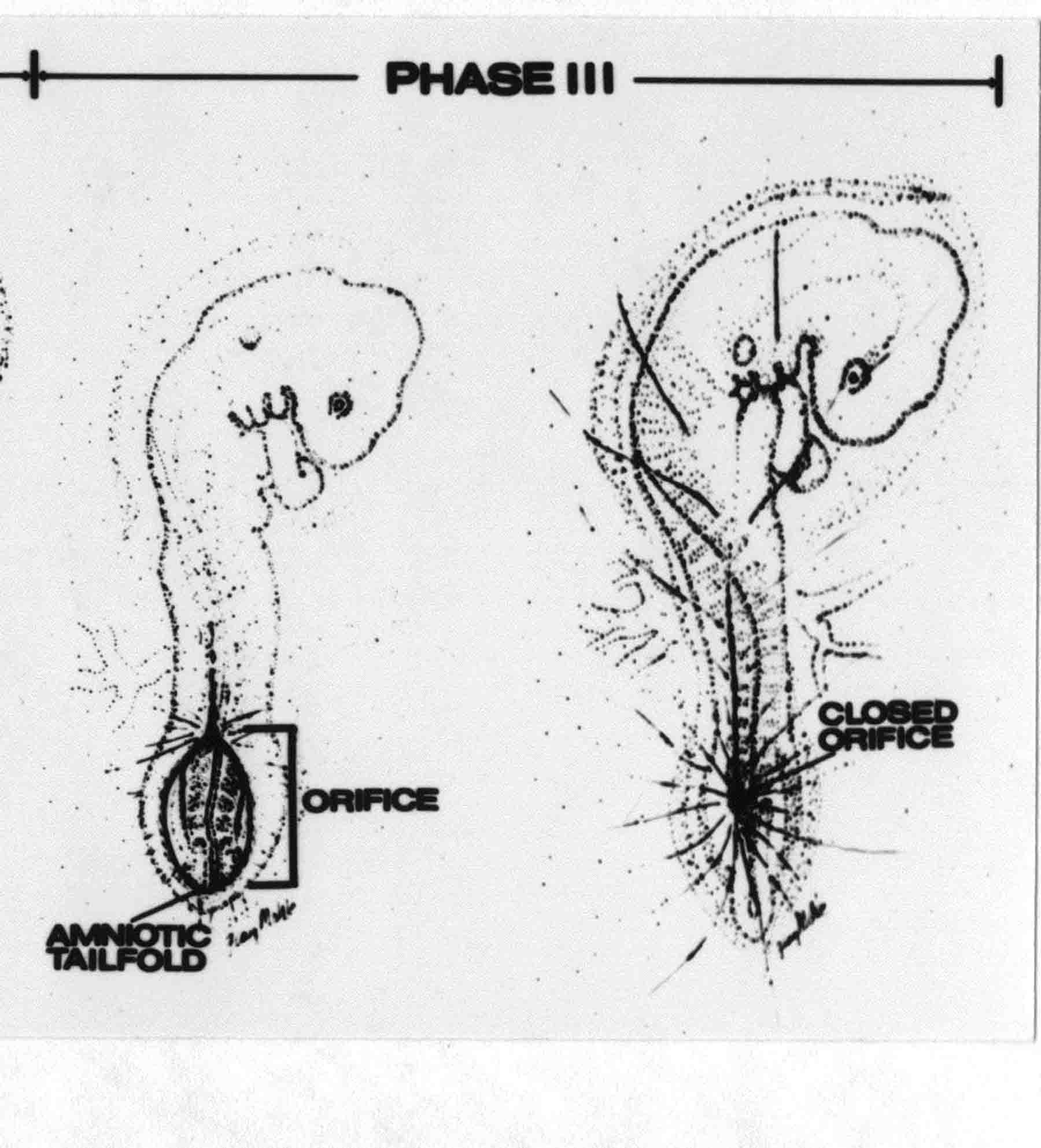 Each phase will now be discussed and illustrated in more detail. The illustrations below show overviews of chick embryos from just before Phase l to the end of Phase l. Scale bars = 0.5 mm. Figure 6 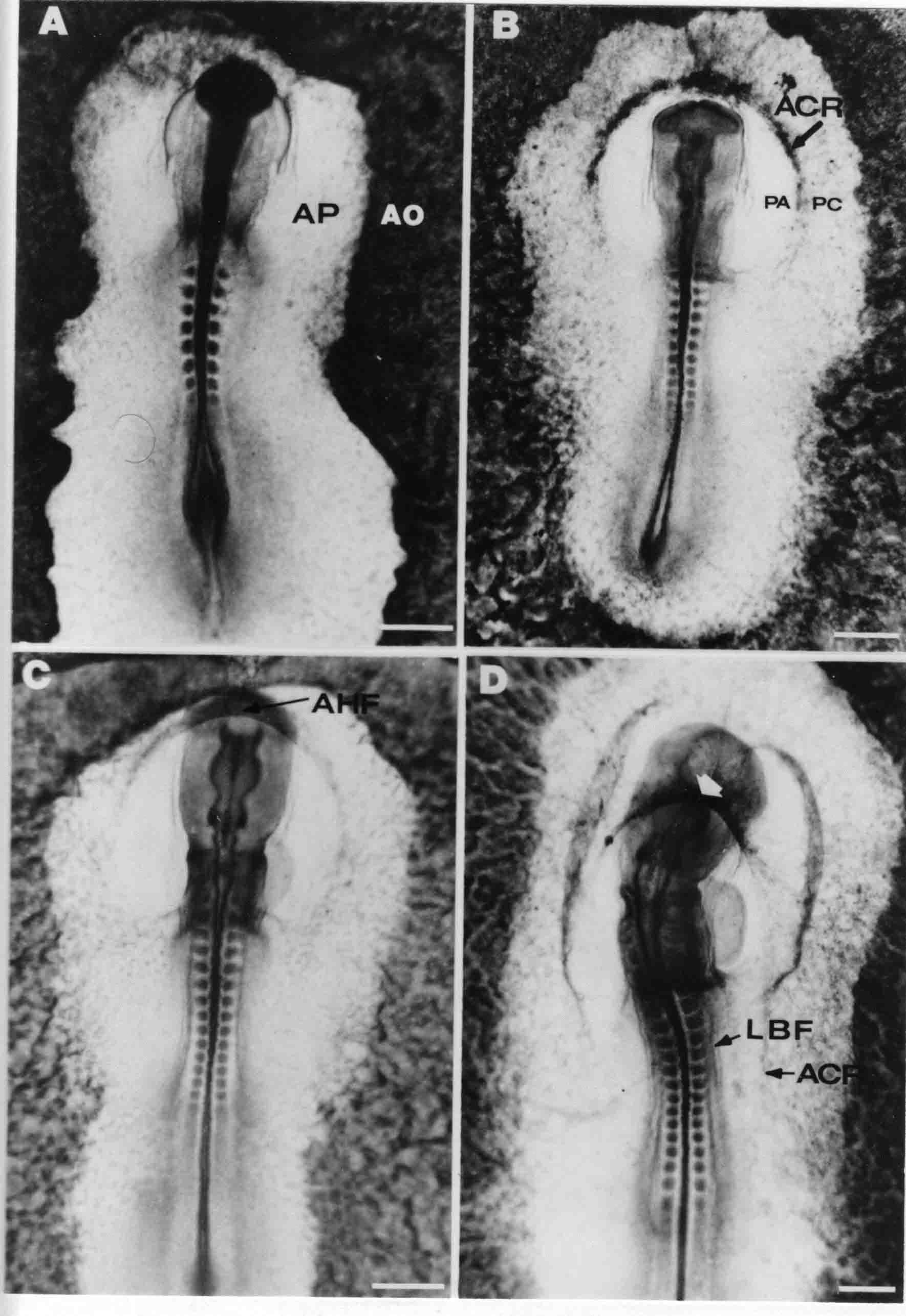 A. In an embryo with 8 somites, stage 9+, there is no overt sign of amnion formation in the area pellucida (AP). AO is area opaca. B. At 13 somites, stage 11, an amniochorionic ridge (ACR) forms a crescent around the head in the area pellucida. The ACR separates prospective amnion (PA) from prospective chorion (PC). C. At 15 somites, stage 12-, the most rostral part of the ACR has become amniotic headfold (AHF) that covers the forebrain. D. At 18 somites, stage 13-, torsion has turned the head onto its left side. The amniotic headfold covers part of the midbrain. The midline of the headfold (white arrow) is denser than in C, but an amniotic delta has not yet formed there. The amniochorionic ridge (ACR) and the lateral bodyfold (LBF) have extended caudally to about the level of the ninth somite. A closer view (scale bar = 0.2 mm) in the illustration below shows ACR cells and beginning AHF in embryos whose cells have been surface-stained with methylene blue. Figure 7 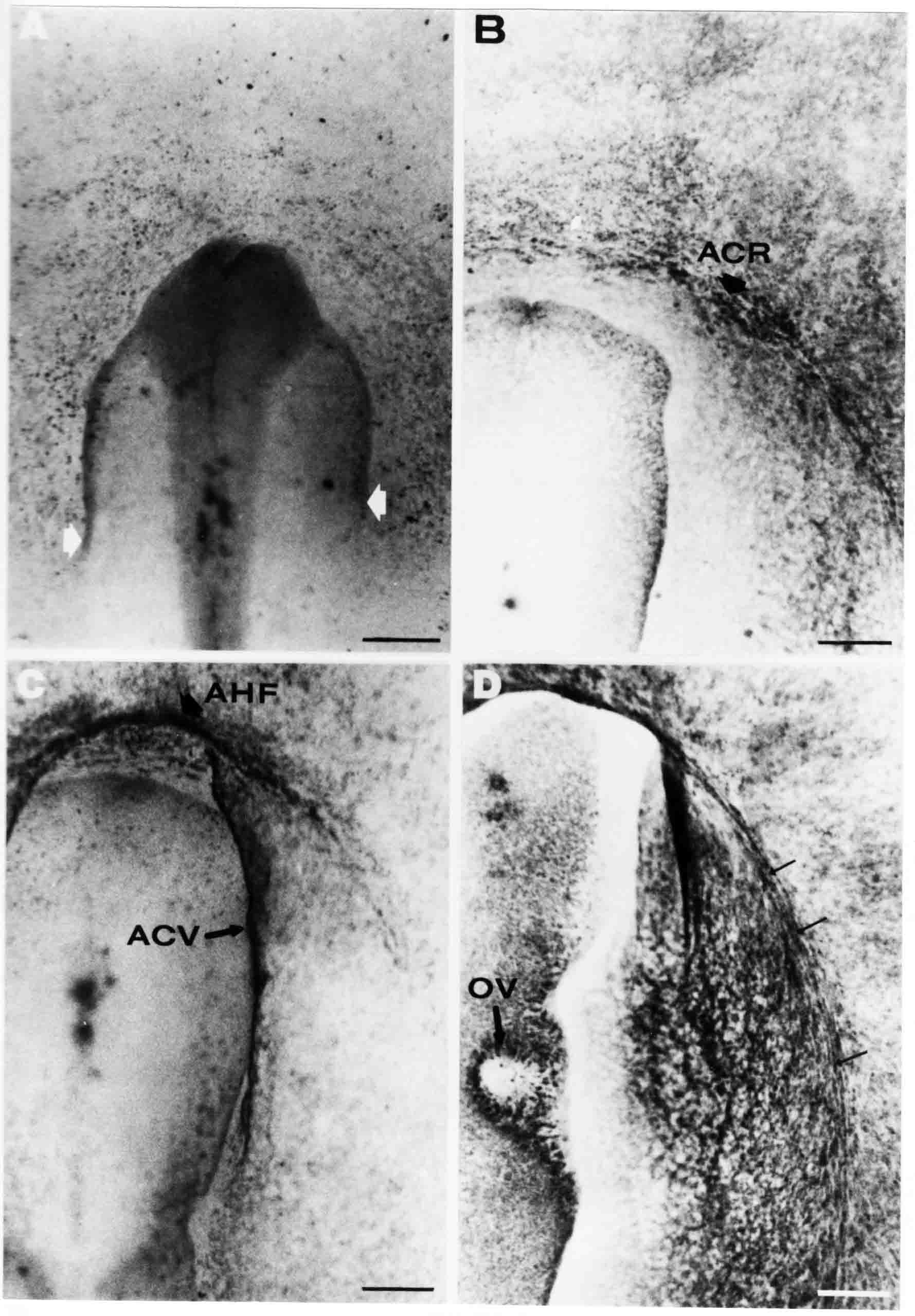 A. In a stage 9+ embryo with eight somites, The extraembryonic ectoderm in front of and to either side of the head show cells that are near isodiametric and show no obvious polarity. Lateral bodyfolds extend to the levels indicated by the arrows. B. In a stage 11- embryo with 12 somites, two distinct cell populations now comprise the extraembryonic ectoderm. Cells of the amniochorionic ridge (ACR) are elongated and arranged in an arc around the head while cells to either side of the ACR are more nearly isodiametric. C. In a stage 11+ embryo with 14 somites, the amniotic headfold (AHF) has formed a small pocket or fold in front of the head. An amniocardiac vesicle (ACV) is seen adjacent to the head. D. In a stage 12+ embryo with 17 somites, the amniotic headfold is over the forebrain and the elongate nature of the ACR cells is seen readily. OV = otic vesicle. Amniochorionic ridges are shown in cross sections from four embryos in the illustrations below. Scale bars = 20 micrometers. Figure 8 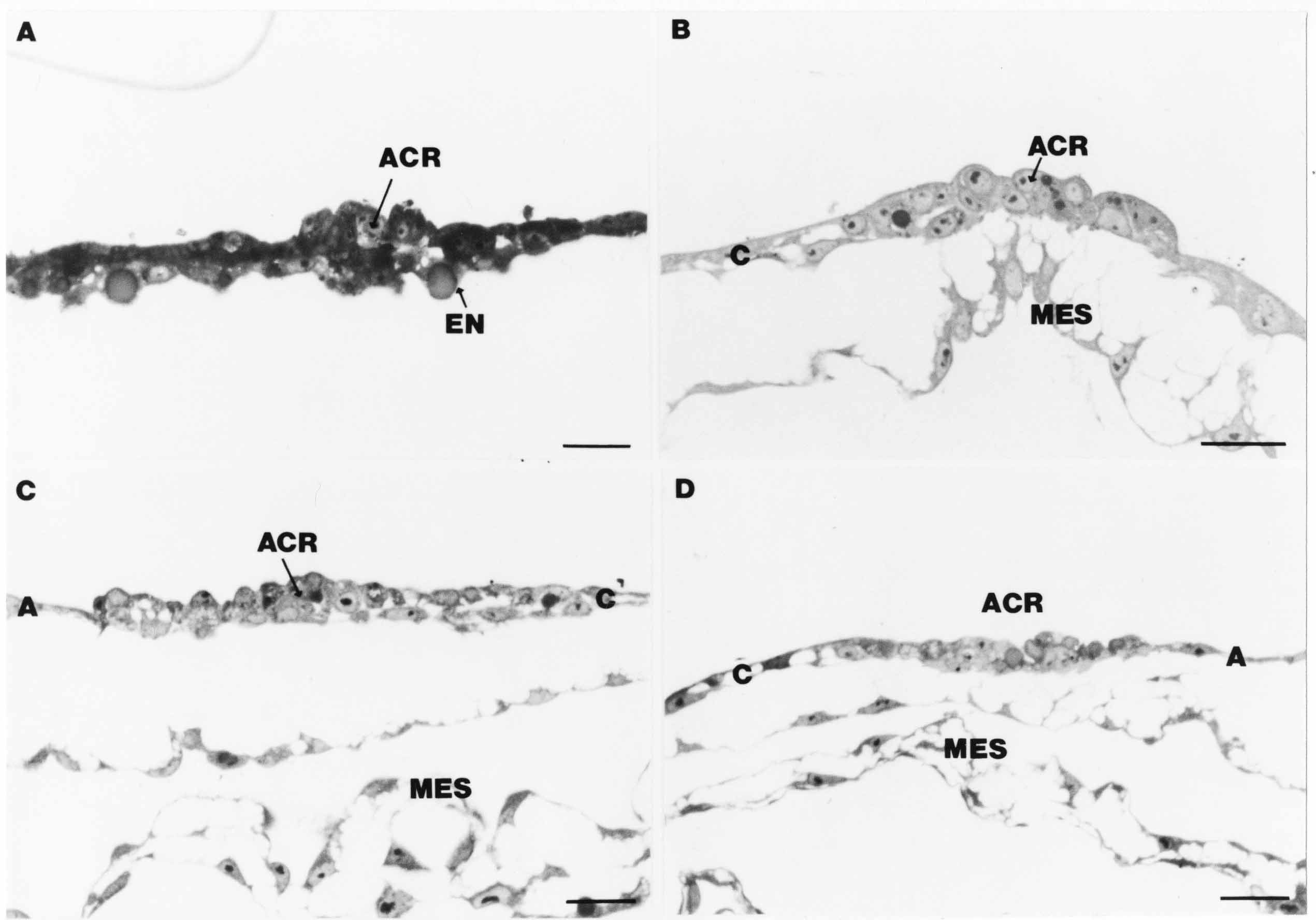 The amniochorionic ridge (ACR) is shown in cross section in these embryos. The ACR occupies the border between amnion and chorion. A. Stage 11-, 12 somites. The mesoderm has not yet migrated between the amniotic ectoderm and the endoderm. B. Stage 11, 13 somites. the somatic mesoderm (MES) has now migrated beneath the ACR. In this section and those following, the chorion (C) is two-layered and the amnion (A) has one layer of ectodermal cells. The ACR cells are cut across their long axes, thus many of these cells are seen in the thick ACR. C. A stage 11, 13 somite embryo, sectioned at a more posterior position. D. Another example of a stage 11 embryo with 13 somites. Phase ll begins about stage 13+. Illustrations below give an overview of this phase. Embryos in A-C have been excised from the yolk and photographed by transmitted light. D is an electron micrograph. Scale markers equal 0.5 mm in A, B, and C, and 0.1 mm in D. Figure 9 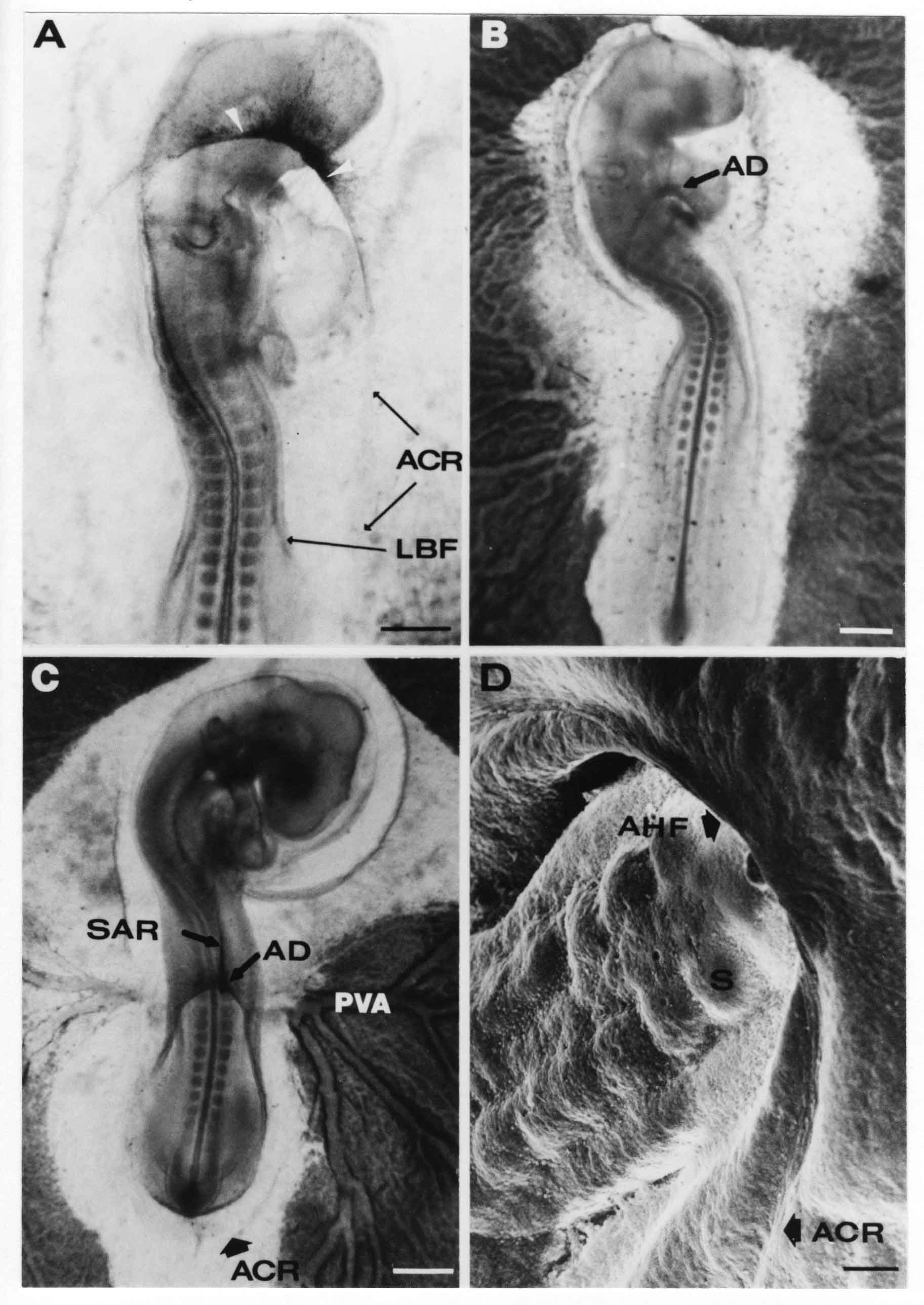 A. The embryo is at stage 13+, with 20 somites. This is the beginning of Phase ll. The midline of the amniotic fold (between white arrows) is much more dense and thickened compared to adjacent lateral amniotic folds. The amniochorionic ridge (ACR) is faintly visible caudally. Its formation keeps pace with the lateral body folds (LBF). B. Stage 14, 21-22 somites. An amniotic delta (AD) has formed at the midline apex of the amniotic headfold. A faint seroamniotic raphe extends rostrally from the delta. The caudal edge of the delta has a smooth broad outline. C. This stage 17 embryo is a very late Phase ll embryo. The amniotic headfold is at the level of the posterior vitelline artery (PVA) The delta (AD) in the amniotic headfold has a nipple or projection of tissue on its caudal face. The amniochorionic ridge (ACR) extends around and behind the tailbud of the embryo. The seroamniotic raphe (SAR) projects rostrally from the amniotic delta. D. This SEM of a late Phase ll embryo shows a close-up of the delta of the headfold of the amnion (AHF). Elongated cells of the headfold line up with elongated cells of the ACR. The illustration below is of Phase ll embryos that have been stained on the surface with methylene blue. Scale markers = 0.25 mm in A, B, and D, and 0.5 mm in C. Figure 10 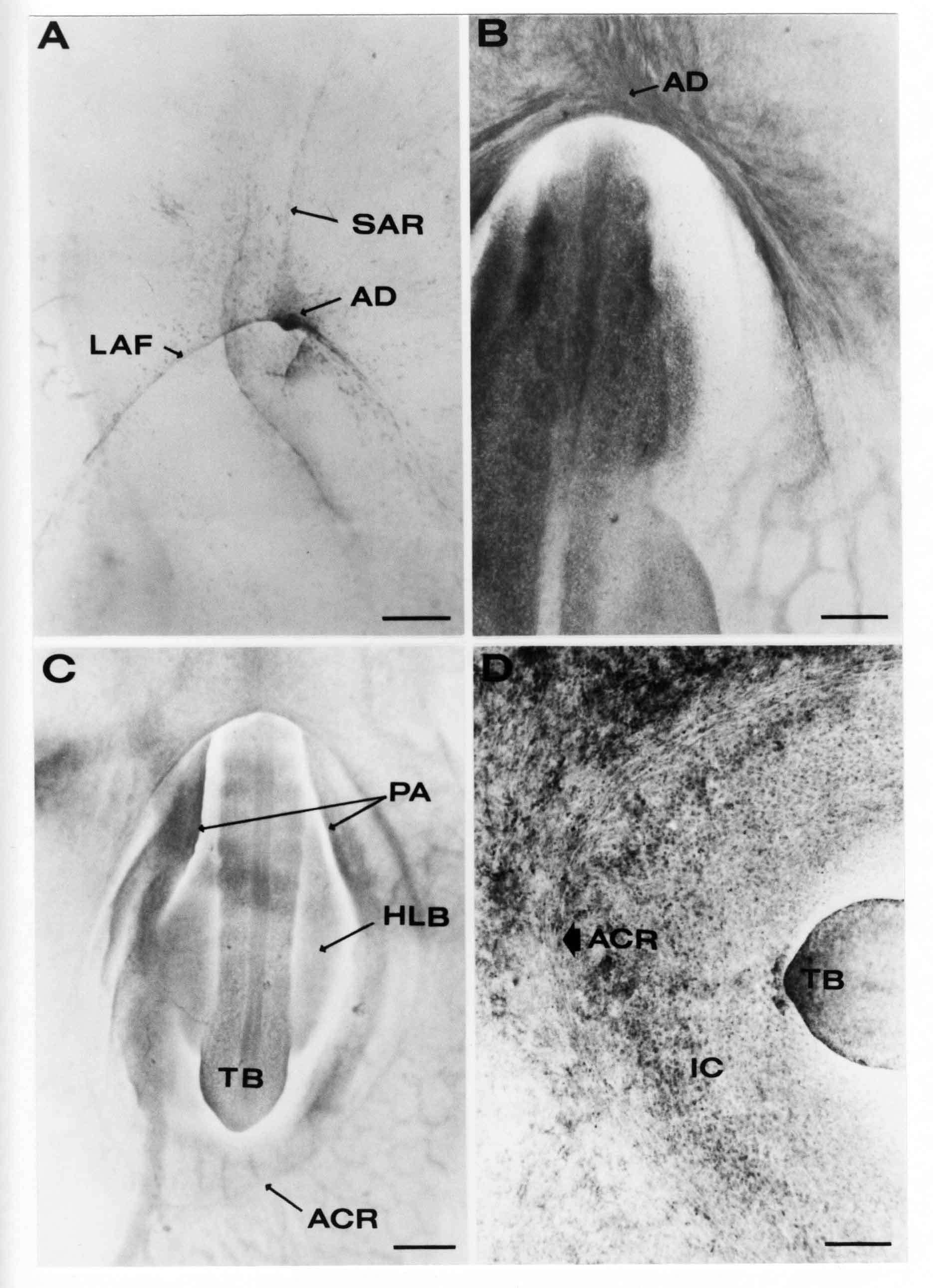 A. The embryo is at stage 15 with 24 somites. The delta shape of the amniotic delta (AD) is readily apparent, as is the nipple on the delta. The seroamniotic raphe (SAR) and the lateral body folds (LAF) also take up the stain. B. An embryo at stage 15 with 25 somites. In contrast to A, the caudal face of this amniotic delta (AD) is smooth; no nipple. C. This stage 17 embryo is a very late Phase ll. The amniochorionic ridge (ACR) has extended around and behind the tailbud (TB). Balooning of the prospective amnion (PA) is indicated. (HLB) is the hindlimb bud. D. This stage 17 embryo shows the amnionic ridge (ACR) behind the tailbud (TB). The cells of the prospective amnion between the ACR and the TB are isodiametric (IC). Phase lll. The tailfold of the amnion forms and lifts over the tail. The lateral amniotic folds of the tailfold fuse with the lateral amniotic folds of the head fold forming the amniotic orifice. The orifice closes to a point. The connection between amnion and chorion is lost. The illustrations below show these changes. Figure 11 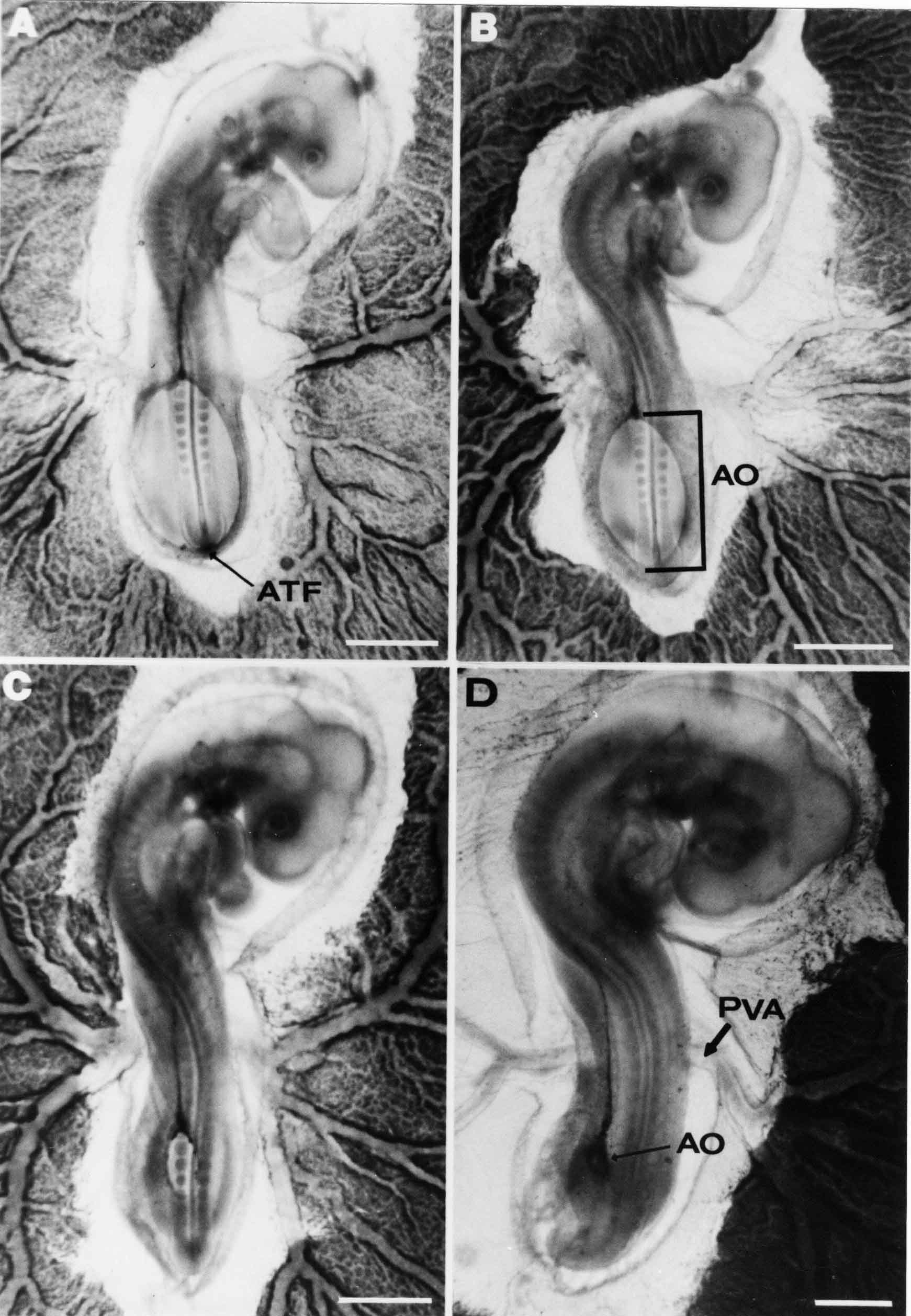 Enbryos in A-D have been excised and photographed in transmitted light. All scale bars = 1 mm. A. An early Phase lll embryo at stage 17. The amniotic tailfold (ATF) has advanced cranially to lie just over the tip of the tailbud. B. A stage 17 embryo slightly older than the embryo in A. All ACR has converted to amniotic fold. The amniotic orifice, encircled by amniotic fold, is now formed. C. In this stage 17-18 embryo, the amniotic orifice has constricted. The tail fold of the amnion is now well craniad to the tailbud. The head fold of the amnion is well caudad to the level of the vitelline arteries where it was in B. Only the head fold possesses an amniotic delta and raphe. D. Stage 18. A very late phase lll embryo. The amniotic orifice (AO) is almost closed off and lies about halfway between the tip of the tail and the level of the posterior vitelline arteries (PVA). More details of the amniotic orifice are shown in the illustration below. Embryos A and C have been surface stained with methylene blue. Embryos B and D were photographed with reflected light after fixation in osmium tetroxide. Scale bars = 0.5 mm. Figure 12 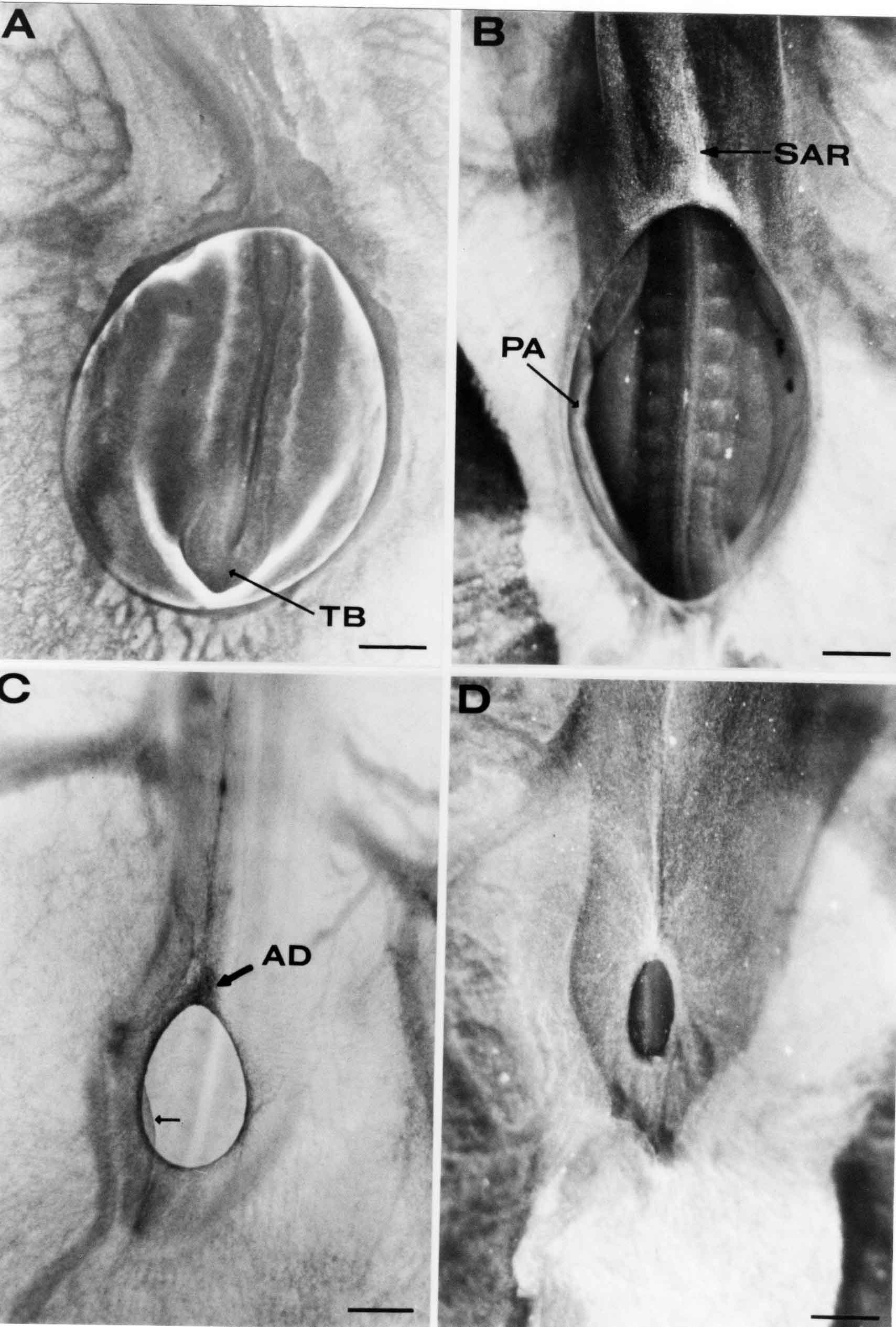 A. In this early Phase lll embryo, the entire edge of the orifice has a smooth outline suggesting that all ACR has converted to amniotic fold. The amniotic tailfold has not lifted above the tailbud (TB). B. This amniotic orifice is slightly older than that shown in A. Some ballooning of prospetive amnion (PA) is seen. The seroamniotic raphe (SAR) extends rostrally from the amniotic delta. C. In this mid-Phase lll amniotic orifice, the orifice has a noose-like appearance. Only the amniotic headfold has an amniotic delta (AD) and amniotic raphe. D. This late Phase lll orifice has the appearance of a pursestring. Cross sections from a Phase ll (stage 15) embryo below show the progression of the process of amnion formation in sections from caudal to rostral. Scale bar = 0.2 mm. Figure 13 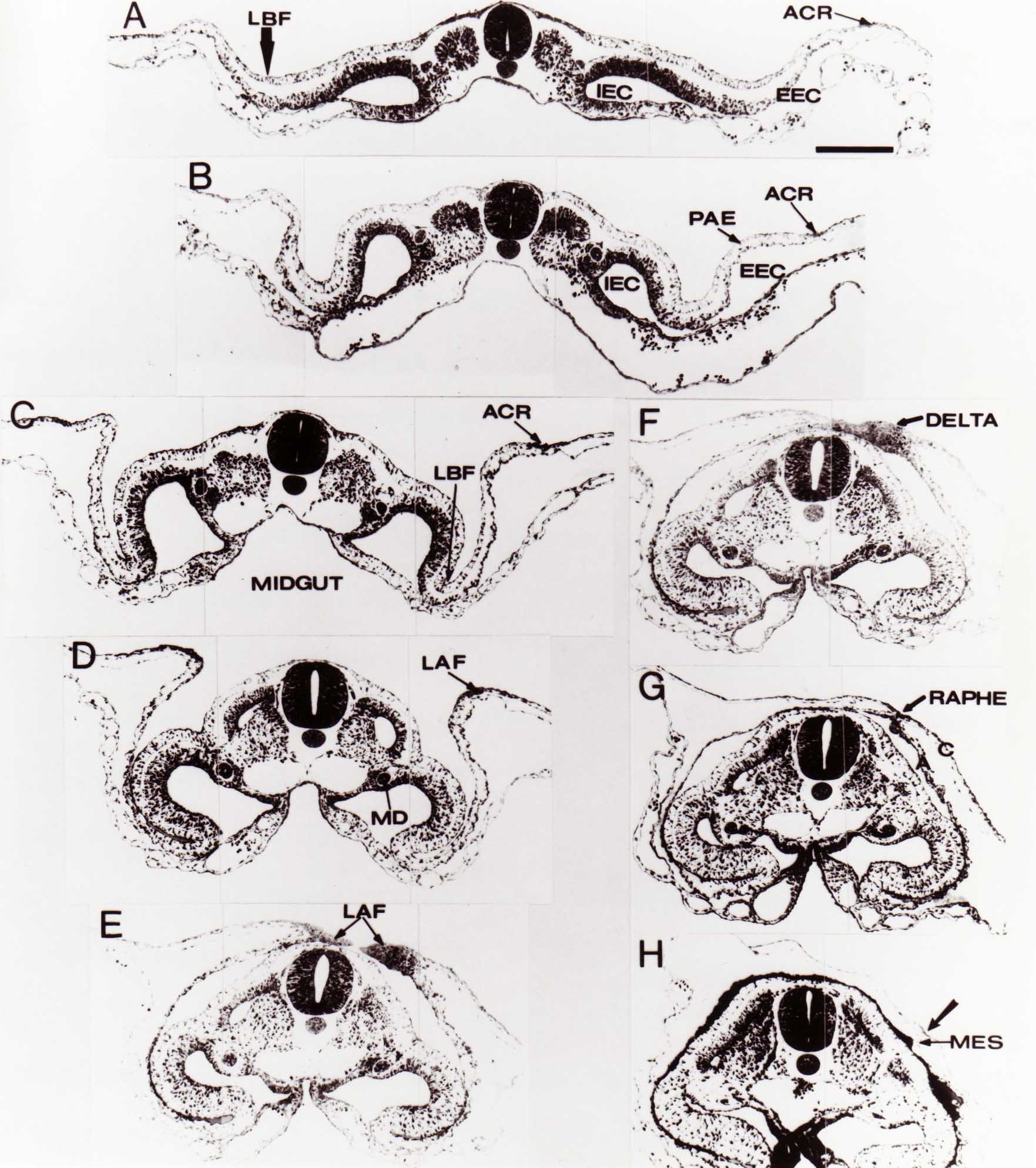 A. This most-caudal of the sections shows the region where the lateral body folds (LBF) are just beginning to form to each side of the embryo. The amniochorionic ridge (ACR) is forming at the same level. The body folds form the boundary between the intraembryonic coelom (IEC) and the extraembryonic coelom (EEC). B. The lateral body folds now protrude downward below the level of the rest of the embryo. The prospective amniotic ectoderm (PAE) is defined to one side by the body fold and to the other side by the ACR. Ballooning of the prospective amniotic ectoderm (PAE) is seen to create a sort of false amniotic fold. The amniochorionic ridge (ACR) will become the actual amniotic fold. C. Lateral body folds (LBF) are starting to undercut the embryo and the midgut endoderm is assuming the shape of a broad inverted V. D. The amniochorionic ridge in now the actual amniotic fold (LAF). Lateral body folds are almost directly beneath the mesonephric ducts (MD). As the body folds pinch towards the midline, the lateral amniotic folds are higher as the embryo appears lower. E. The section at this level shows the lateral amniotic folds (LAF) about to fuse above the embryo. F. This section is through the amniotic delta just rostral to the union of the two amniotic folds. The midgut has a prounced inverted V shape. G. This section is rostral to the amniotic delta and through the seroamniotic raphe. The raphe is here just a small ectodermal strip connecting the amnion (A) and the chorion (C). H. This secton is a little more rostral than G. The seroamniotic raphe has broken down completely. The chorion has a persistent thickening where the site of the raphe had been (black arrow). Amniotic mesoderm (MES) extends unbroken across the position where the raphe had been. The above material provides a better description of amnion formation than had existed before. The material that follows takes a closer look at the cellular nature of amniochorionic ridge, amniotic fold, amniotic delta, seroamniotic raphe, and amniotic orifice. The illustration below shows transmission electron micrographs of sections through the amniochorionic ridge. Scale bars = 1 micrometers. Figure 14 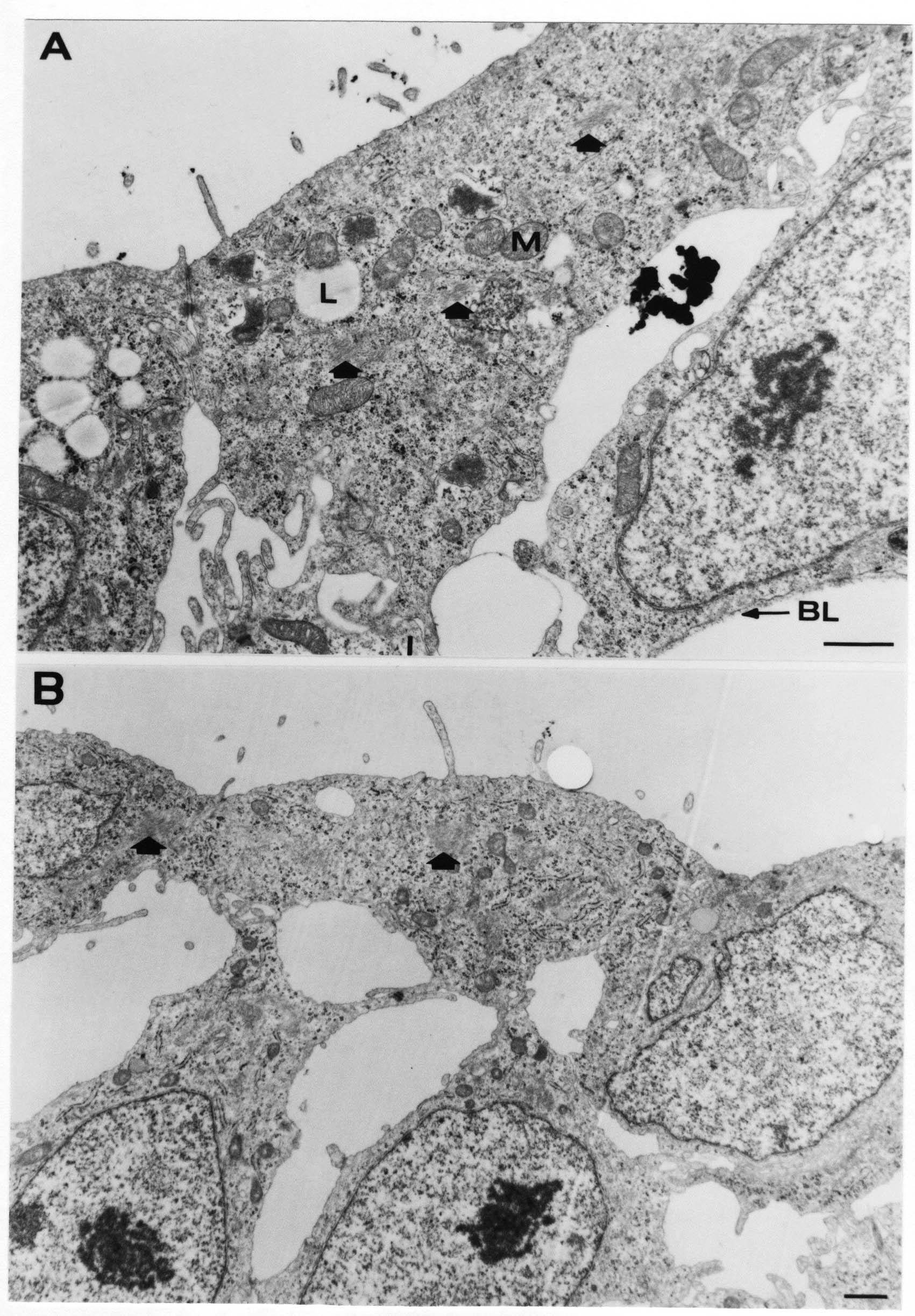 A. This is a cross-section through the amniochorionic ridge of a thirteen somite chick embryo (Stage 11). Numerous mitochondria and lipid droplets are present in the cytoplasm. Several microfilament bundles (arrows) are seen. BL = basal lamina. B. Cross-section through the amniochorionic ridge of a stage 12- embryo. Arrows indicate microfilament bundles. The illustration below shows intracellular details in transmission electron micrographs of sections through amniotic folds, Scale bars for A-D = 0.2 micrometers. Scale bars in both insets = 0.5 micrometers. Figure 15 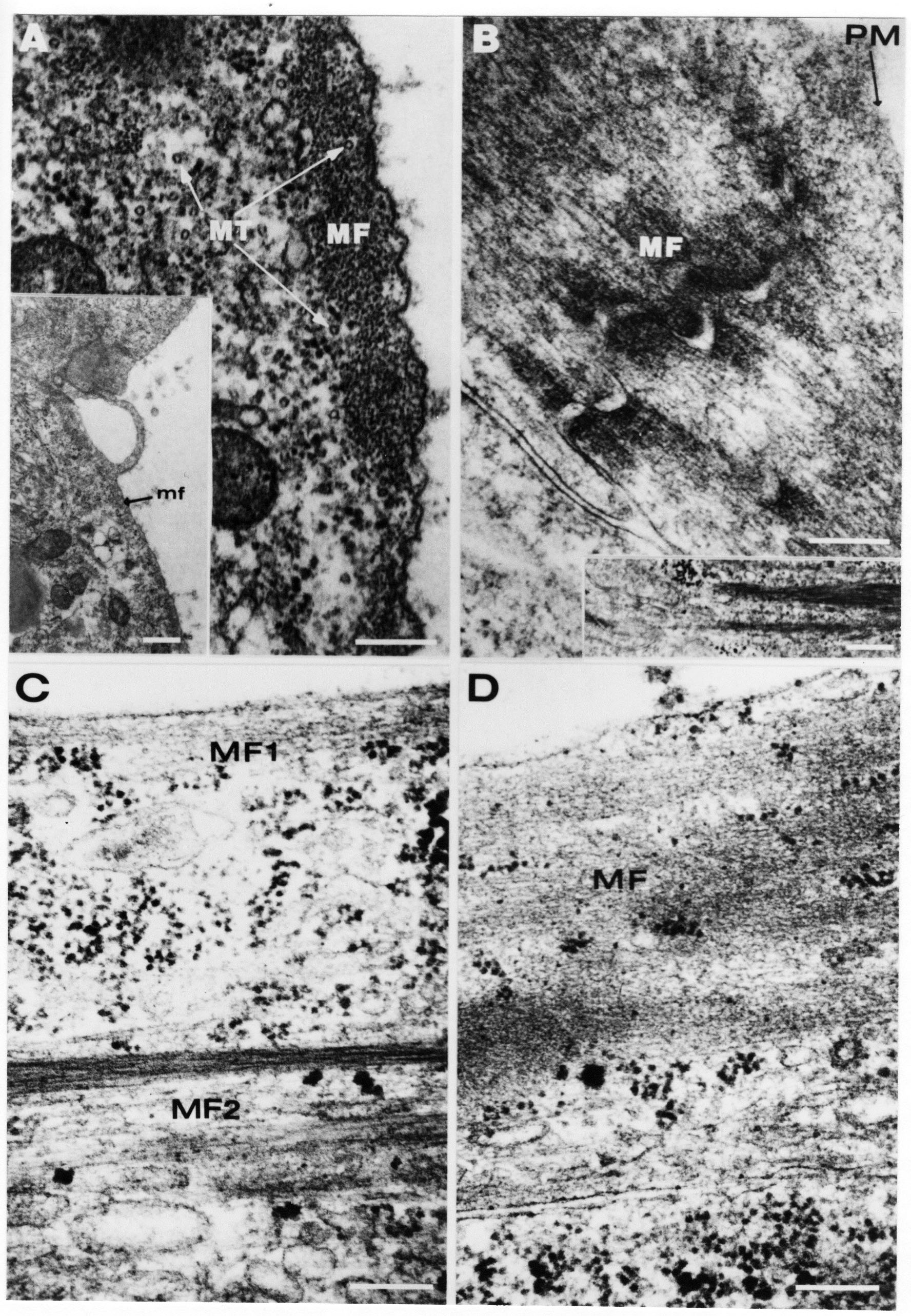 A. Sagittal section through the amniotic headfold of a 15 somite embryo. A layer of microfilaments (MF), cut in cross section, underlies the plasma membrane of the ectoderm. Numerous microtubules (MT) are interspersed among and below the layer of microfilaments. Inset: the layer of microfilaments is seen at lower magnification. B. Frontal section of a section through the amniotic headfold of a 15 somite embryo. Numerous microfilaments are seen in longitudinal section and terminate at the junction of two cells. The apparent density of the microfilaments is increased at the region of the cell junctions. Inset: an example of microfilament bundles often seen deeper in the cytoplasm. C. Frontal section through the amniotic headfold of a stage 13-14 embryo. Some microfilaments (MF1) are located immediately subjacent to the cell membrane. Other microfilaments (MF2) are located deeper in the cytoplasm. D. Another example of microfilaments from the same embryo used for the section in C. Numerous microfilaments are seen in approximately longitudinal profile. The TEM (transmission electron micrograph) below shows saggital sections through an eighteen somite embryo at stage 13-. Scale bar in A = 2 micrometers, 1 micrometer in B, and 0.2 micrometers in C. Figure 16 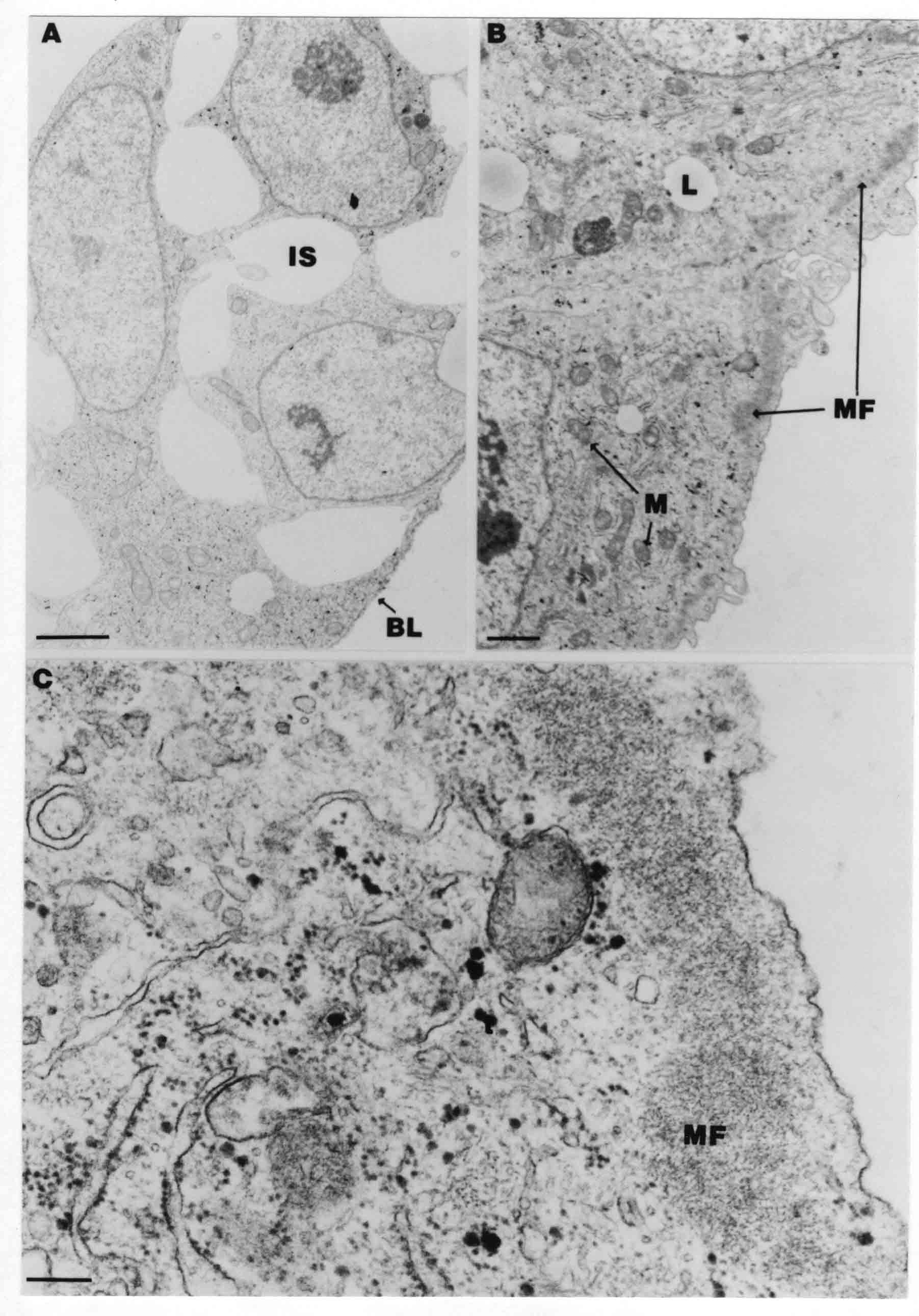 A. TEM through the head ectoderm. The basal region is underlain by a lamina (BL). Apically there is no microfilament layer as is seen in the amniotic fold. Numerous intracellular spaces (IS) pervade the body ectoderm. B. TEM through the tip of the amniotic fold. Several regions of subplasmalemmal microfilaments (MF) may be seen. Numerous mitochondria (M) and lipid droplets (L) are present. A portion of this micrograph is seen at higher magnification in C below. C. In this TEM at higher magnification through the tip of the amniotic fold, a band of mirofilaments (MF) approximately 0.5 micrometers wide is seen beneath the plasma membrane. The bundle of microfilaments excludes other cytoplasmic organelles. The microfilaments appear to be cut in cross-section. The TEM's below show Phase ll of amniotic fold formation. Figure 17 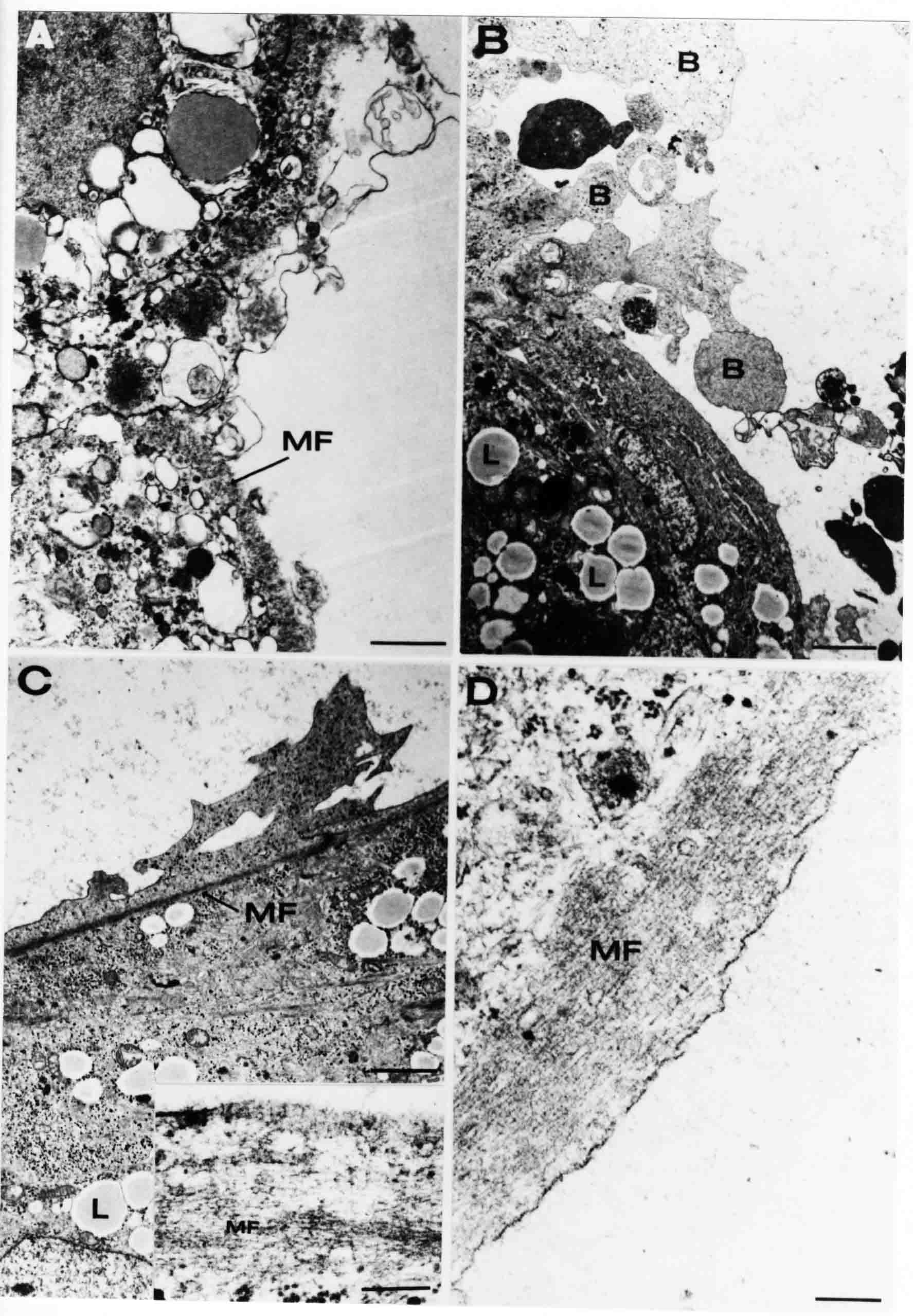 A. Sagittal section through the amniotic fold of a glycerinated stage 13-14 embryo. A meshwork of microfilaments (MF) is seen at the edge of the cells. Scale bar = 1 micrometer. B. Stage 14-15. Low magnification frontal section through the nipple on the caudal face of the delta. Considerable blebbing (B) is seen, as are numerous lipid droplets (L). Scale bar = 2 micrometers. C. Frontal section through the lateral amniotic folds adjacent to the region shown in B. A band of microfilaments (MF) underlies the plasma membrane. Numerous microfilaments are seen in longitudinal profile. Inset scale bar = 0.2 micrometers. D. Longitudinal section through the lateral amniotic fold of a late Phase ll embryo (Stage 16). A large zone of microfilaments (MF) creates an organelle-free border. These microfilaments are cut in approximately longitudinal section. Scale bar = 0.25 micrometers. The TEM's below show Phase ll of amniotic fold formation in a stage 14-15 embryo. Scale bar = 0.2 micrometers in A, and 0.1 micrometer in B. Figure 18 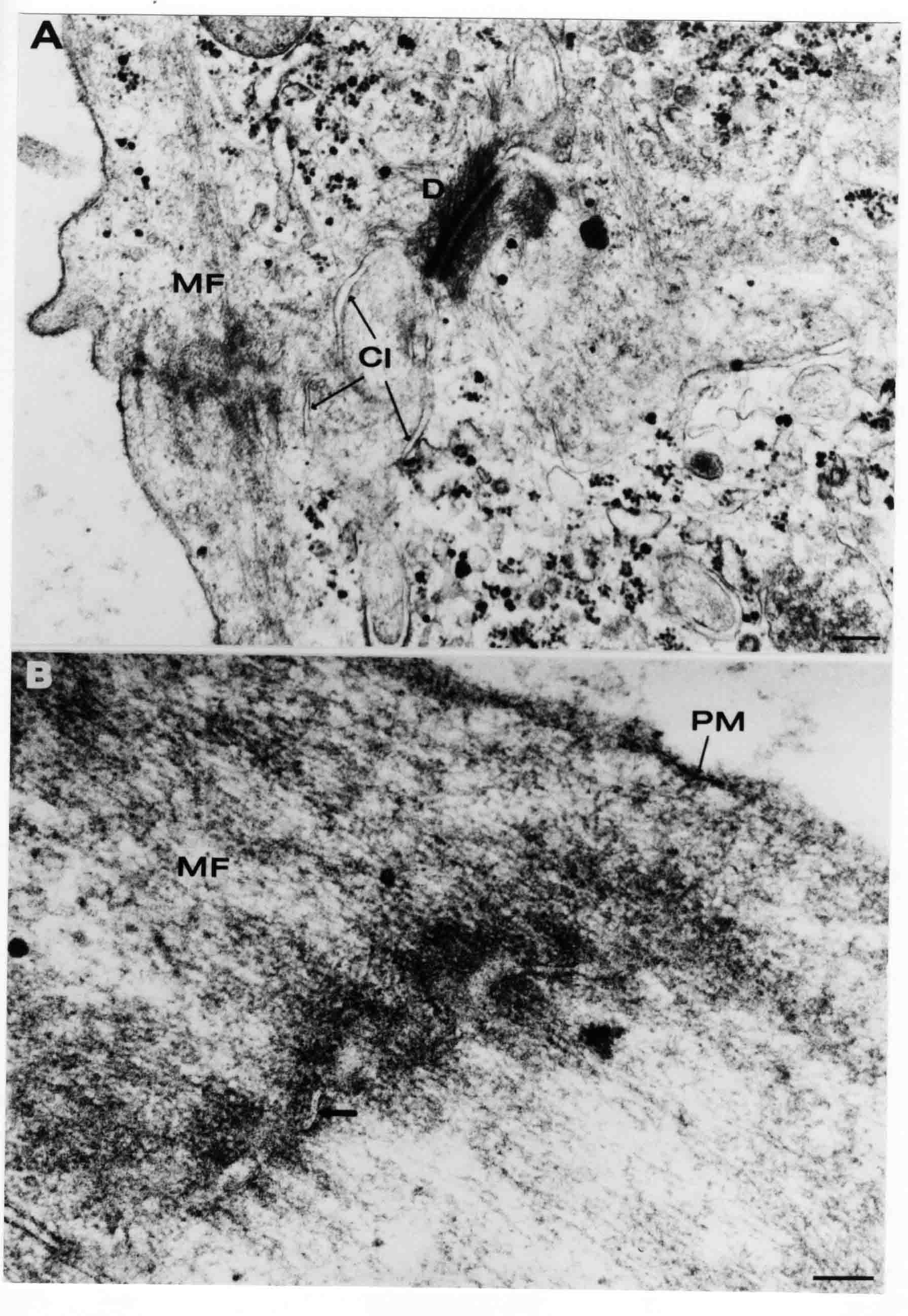 A. Frontal section through the lateral amniotic fold illustrating the commonly seen arrangement of a zone of microfilaments (MF), a region a cell interdigitation (CI), and a desmosomal region (D). B. Higher magnification of the microfilament region of the apical junction. The region below the plasma membrane is filled with microfilaments (MF) inserting into the plasma membrane (large arrow) of two adjacent cells. The apparent density of the microfilaments is greatest at the cell junction. The TEM's below are of Phase III of amniotic fold formation. Figure 19 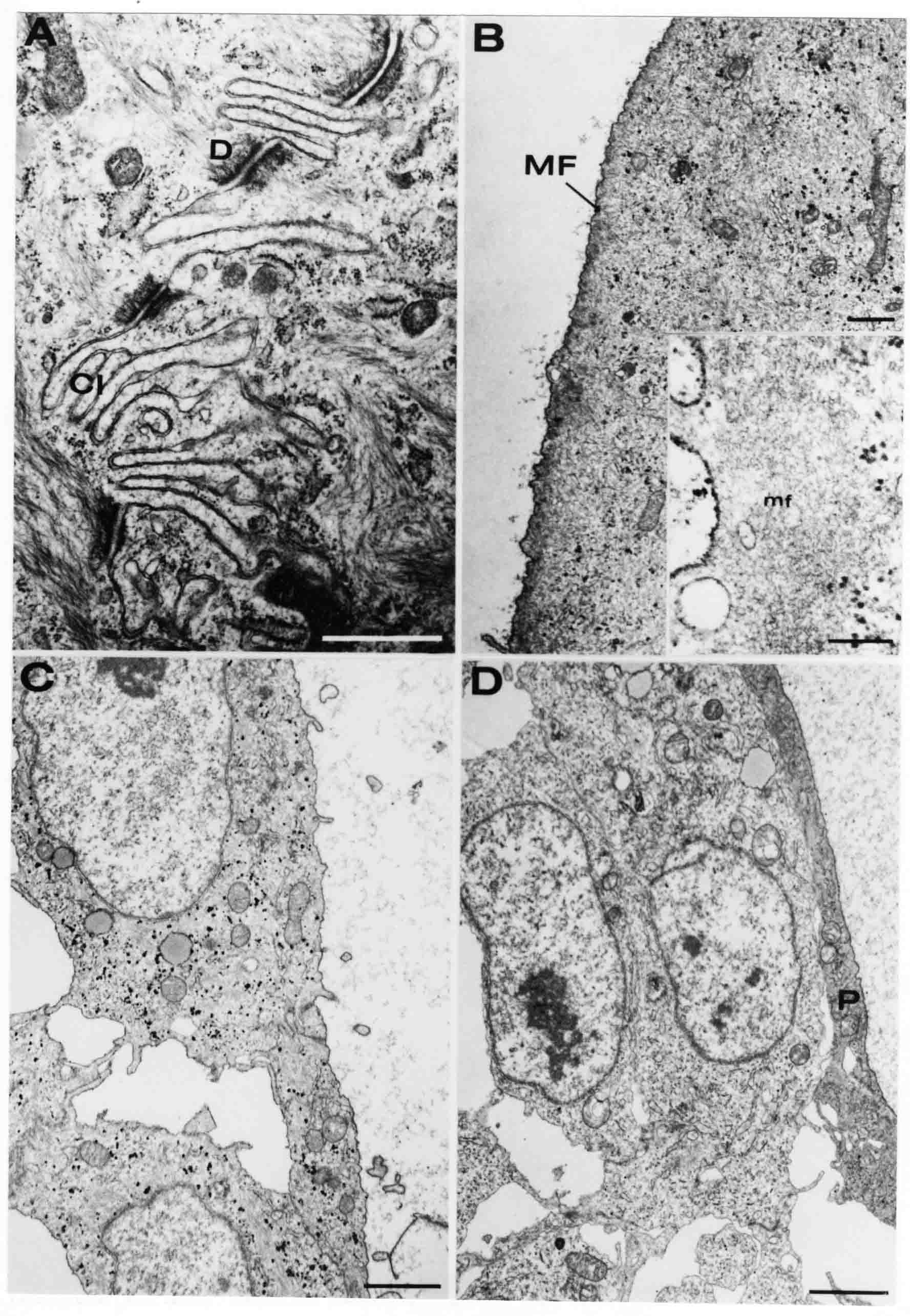 A. Parasagittal section through an amniotic orifice shortly before closure. Considerable cell-cell interdigitation is shown. Numerous desmosomes (D) are present at intervals between regions of interdigitation. Most of the cytoplasmic filiments are seen to insert into the desmosomes. Scale bar = 1 micrometer. B. Cross section through an amniotic orifice. A zone of microfilaments C. Non-fold amnion of a Phase III embryo. This ectoderm lacks the microfilament border seen in the amniotic fold. Scale bar = 2 micrometers. D. An example of the peridermis differentiation in the body ectoderm. The peridermis (P) forms a dark-staining layer over the underlying ectoderm. Scale bar = 2 micrometers. In this descriptive section, two questions can be asked: (1) How is the amniochorionic ridge (ectamnion of Lillie (1903)) formed? (2) what are the natures of the forces responsible for the craniocaudal movement of the amniotic headfold, the formation of the tailfold, and the closure of the amniotic orifice? Phase I In embryos with 7-8 somites (stage 9) there is no overt morphological differentiation of the extraembryonic ectoderm demonstrable by surface staining of the ectoderm (Figs 6A and 7A) and the extraembryonic ectoderm also appears morphologically homogenious in sectioned embryos of that stage. However, by 9 somites (stage 10-) two distinct cell types can be distinguished in the area pellucida ectoderm in front of the head (Fig.7B). The majority of cells are nearly isodiametric, that is, their lengths and widths are similar. Within the area pellucida is a second cell population arrayed in the form of a horseshoe; the open end of the horseshoe extends to the level of approximately the third or fourth somite pair. The cells of this horseshoe are elongate with their long axes directed along the arc of the horseshoe. Lillie (1903) applied the term "ectamnion" to this arc of cells; I prefer the term "amniochorionic ridge" as it more fully describes the ridge-like morphology of the cells as seen in cross-section (8A-D) and the position of the cells within the blastoderm. Amniochorionic Ridge Formation of an amniochorionic ridge (ACR) precedes formation of amniotic fold. The ACR progressively transforms into amniotic fold. The ACR forms first as an inverted U-shape around the head of the embryo (Figs. 3, 6B). As this portion of ACR converts to amniotic fold, new ACR arises lateral to each side of the embryo, progressing posteriorly in concert with formation of the lateral body folds (Figs. 6C,D;9A.) The ACR is a distinct line of cells that differ in appearance from the forming amnion and chorion to its sides. Viewed looking down on the apical surfaces of these squamous cells, the cells of amnion and chorion appear isodiametric, while the ACR consists of a band of elongated cells (Figs. 7B,C,D;10D). In cross section, the ACR appears more densly packed than adjacent chorion and amnion cells; the packing of the cells forms the ridge. Ridge cells are rounded up in cross section compared to the flatter cells of amnion and chorion to either side (Figs. 8A-D). The most-cranial ACR forms before the mesoderm has migrated between ectoderm and endoderm (Fig. 8A), suggesting that the mesoderm has little or no role in the shaping of the ridge. Amniotic Fold The amniochorionic ridge cells convert to amniotic fold cells in a craniocaudal progression. At approximately 14 to 15 somites (stage 11+) the conversion of amniochorionic ridge into the headfold of the amnion begins to occur. The first indication of headfold formation is the appearance of a pocket or fold in the midline in close proximity of the head of the embryo (Fig. 7B). As the headfold begins its caudally-directed movement over the embryo the amniotic fold extends somewhat farther posterolaterally on either side of the head. By 15 somites (stage 12-) the headfold is normally found over the level of the prosencephalon (forebrain) (Fig. 6C). In slightly older embryos (Fig. 6D), the midline apex of the headfold becomes more dense in appearance. No indication of a seroamniotic raphe is seen in either surface-stained or cross-sectioned embryos at these stages (Fig. 6C). At the same time that the amniochorionic ridge is being transformed into amniotic fold, two other events pertinent to amniotic fold morphogenesis occur. Cranial flexure flexes the head ventrally while torsion rotates the head onto its left side (Fig. 6D). In addition, there is an expansion of the amniocardiac vesicles (the extraembryonic coelom at the level of the heart). The expansion is usually difficult to preserve in fixed and sectioned embryos, but may be readily seen in Auerbach cultures, especially if viewed from an angle with a dissecting microscope (see Fig. 7C). By stage 12, there are three components to the amniotic fold: the amniotic headfold overlying the head of the embryo in the midline, the lateral amniotic folds extending posteriorly and laterally from the headfold, and the more posteriorly located amniochorionic ridge that has not yet become part of the lateral amniotic folds. The posteriormost cells of the amniochorionic ridge are "splayed out" in the amniotic ectoderm (Fig. 7D). When the headfold of the amnion is first formed, it is little more than a fold (Fig. 6C); as it moves caudally from the tip of the head to approximately the level of the otic vesicles, the leading edge of the headfold increases in width and thickness (Fig. 6D). There is a gradual change in the shape of the leading edge of the fold so that the midline apex acquires a cuneiform or delta-like shape (Figs. 10A, 10B). I have termed this the amniotic delta. The seroamniotic raphe, a transient connection between amnion and chorion (Figs. 9B, 9C, and 13G) will extend rostrally from the amniotic delta during phases ll and lll. Phase II The second phase of amniotic fold formation commences once a delta is visible at the apex of the headfold; this has generally occurred by stage 13+. During phase ll, the headfold continues to move caudally over the back of the embryo and as it does so, several features are seen that are relevant to the process of amniogenesis. As the headfold moves caudally, the amniochoronic ridge continues to form at progressively more posterior levels on both sides of the embryo. By the time the headfold has reached the level of the posterior vitelline arteries (somite 22), the amniochorionic ridge has extended behind and around the tailbud. The disposition of the ridge is illustrated in embryos that have been stained with methylene blue (Figs. 9C, 10C and 10D). The posterior movement of the headfold is accompanied by the appearance of the seroamniotic raphe and by a change in the overall morphology of the amniotic delta. At the beginning of phase ll, the outline of the headfold and the lateral amniotic folds is broad and smooth (Fig. 9B). This morphology is generally maintained until the headfold reaches approximately the level of the heart ventricle; at about that time a nipple of tissue appears on the posterior face of the amniotic delta so that the outline is no longer uninterrupted (Figs. 9C,10A). The existence of this nipple of tissue seems to be transitory, however, as it is generally not seen later in development (Figs. 9D, 10B and 10C). The seroamniotic raphe extends anteriorly from the amniotic delta during phase ll (Figs. 9C and 10A). The raphe is a transient connection between amnion and chorion; this connection is most massive in the amniotic delta and becomes progressively attenuated at more anterior levels until the amnion and chorion are free from one another (Figs. 13F, 13G and 13H). In the embryos in which the chorion has been stained with methylene blue, the raphe is seen in surface view as a population of lined up cells (Figs. 10A and 10B). It is not known whether the amnion, like the chorion, also has lined up cells at the position of the raphe. In this respect, it is interesting that a persistent thickening is found in the chorion, but not in the amnion (Fig.13H), after breakdown of the seroamniotic raphe. During phase ll there is a "ballooning" or folding of the extraembryonic ectoderm slightly in advance of the headfold. The result of this ballooning is the creation of a sort of false amniotic fold (Fig. 9D). The false fold is located in that prospective amniotic ectoderm between the lateral body fold and amniochorionic ridge; the true limbs of the amniotic fold (the lateral amniotic folds) arise from the more laterally situated amniochorionic ridge. Another feature of phase ll is the close correlation in the most-posterior development of the amniochrionic ridge and that of the lateral body folds. This correlation is also seen in phase l, but is more readily noticed in phase ll. The relationship is difficult to visualize in embryos excised from the yolk, but is readily seen in surface-stained embryos where it was first noticed (Fig. 9A). In those embryos in which the headfold is delayed for some unknown reasons, the amniochorionic ridge is much further differentiated posteriorly than it would be if one considered only the position of the headfold. This correlation is seen also in serially sectioned embryos: the amniochorionic ridge becomes recognizable at levels at which the lateral body folds also begin to appear (Fig. 13A). Phase ll is considered to be completed once the amniochorionic ridge has extended around the tailbud. At this time, all of the amniochorionic ridge has been formed and during phase lll the ridge will be converted into amniotic folds. The end of phase ll is usually at about 60 hours of incubation or about stage 17. Phase III During phase lll the amniochorionic ridge behind the tailbud is elevated as the amniotic tailfold. The combination of headfold, tailfold and lateral amniotic folds forms the amniotic orifice. The orifice then closes off to complete formation of the amniotic sac. Closure is generally complete by stage 18. The disposition of the amniochorionic ridge that will form the amniotic tail fold is quite similar to that of the amniochorionic ridge that forms the amniotic headfold during phase l. The amniochorionic ridge is seen in surface-stained embryos as a strap of elongate cells located behind and around the tailbud (Fig. 10D). As the tailfold is formed and moved up over the tailbud, neither delta nor raphe is seen (Fig. 12C). The presence of a delta and raphe associated with only the headfold portion of the orifice lends an asymetry to the orifice and the overall appearance of the orifice is similar to that of a noose or necktie (Figs. 12C and 12D). As soon as the amniotic orifice is formed it begins to decrease in diameter and soon closes off. The process of closure proceeds simultaneously from both ends and sides of the orifice so that headfold moves farther posteriorly while the tailfold moves farther anteriorly. As it closes off, the orifice has a pursestring appearance. Cells constituting the lip of the orifice are arranged approximately circumferentially around the orifice, whereas those cells in the chorion lateral to the lip of the orifice are radially arranged (Fig. 12D). As was the case in phases l and ll, closure of the amniotic orifice is accompanied by some ballooning of the amniotic ectoderm (Figs. 12B and 12D). The impression one gains from observing many orifices at various stages of closure is that the amniotic folds are somewhat more rigid than the amnion proper and reinforces the appearance of the orifice as a net- or pursestring-like structure. Electron Microscopy of Amniotic Fold Formation Electron microscopy was undertaken primarily to determine whether the ultrastructure would suggest how the posterior movement of the amniotic headfold was accomplished. The chick ectoderm can be divided into five general regions. From the midline laterally, these regions are: (1) the body ectoderm, (2) the lateral body fold (the border between embryonic and extraembryonic ectoderm), (3) prospective amniotic ectoderm, (4) amniochorionic ridge or amniotic fold (depending upon level of section), and (5) prospective chorionic ectoderm. The embryonic body ectoderm is arranged as a bilayer in which there is considerable intercellular space (Fig. 16A). The outermost layer is peridermis while the basal layer is prospective epidermis (Sengal, 1975); at these stages there are no peridermal granules to distinquish the two. The overall height of the two layers of the body ectoderm is considerable except over the neural tube where it is compact and more squamous; the latter appearance is presumedly a result of fusion of opposite levels of ectoderm after neural tube closure. The body ectoderm is continuous with that of lateral body folds. There are no obvious morphological specializations that distinguish lateral bodyfold ectoderm from the ectoderm on either side. The position of the bodyfold is indicated by the folding. The prospective amniotic ectoderm has a proximo-distal gradation in height. Adjacent to the bodyfold the ectoderm is bilayered and of the same height as the bodyfold whereas adjacent to the amniotic fold the ectoderm is still bilayered, but the overall height is decreased and the two layers are more squamous. In younger embryos (8 to 13 somites) the prospective amnion in the region of the proamnion is a monolayer rather than a bilayer. The amniochorionic ridge and the amniotic folds differ from other regions of the ectoderm in that both seem to represent a modification or expansion of the outermost layer. No layer of cells has ever been onserved to cover either of these structures. The fifth region of the ectoderm, the prospective chorion, is clearly a bilayered, squamous cell layer. The transition from amniotic fold to chorion is well defined and there appears to be no gradation in thickness of the chorion as in the amnion (Fig. 8D). All of the extraembryonic ectoderm as well as the body ectoderm is underlain by a basal lamina at this time. The basal lamina separates the ectodermal component of the somatopleura from the mesodermal component. At the ultrastructural level a number of features characterize the amniotic folds and make these cells distinctive as compared to other cells of the ectoderm. Most prominent, and used most often to identify the amniotic folds in electron micrographs, is the arrangement and characteristics of the apical junctional complexes in the amniotic fold cells. A representative junctional complex is illustrated in Figure 18A. At the apical region of the cell there are close or tight junctions with associated bundles of microfilaments. Below this region is considerable cell-cell interdigitation, and below that a region of conventional desmosomes with associated tonofilaments. (In some instances the desmosome region has been observed to be located more apically than the interdigitated region.) The bundles of apical microfilaments create a subplasmalemmal region that is usually devoid of other cytoplasmic organelles. The depth of this region is approximately 0.2-0.5 microns. The microfilaments measure about 7.1 nanometers while desmosome-associated tonofilaments were about 10.0 nanometers in diameter. At the same magnification it was clear that the tonofilaments were larger than the microfilaments. Some embryos were glycerinated according to the method of Ishikawa et al. (1969) in an attempt to remove some of the cytoplasmic components. A network or mesh of apical microfilaments is seen in these embryos (Fig. 17A). Apical microfilaments are a consistent feature of all three phases of amniotic fold formation. The microfilaments are oriented such that their long axes are directed along the long axes of the amniotic fold cells. During phase l, a sagittal section through a stage 11+ embryo in which the headfold is over the prosencephalon reveals a network of microfilaments beneath the plasma membrane (Fig. 15A). The filaments are cut in cross-section and appear to be more numerous at cell junctions. Numerous microfilament profiles are also seen. When a similarly staged embryo (Fig. 15B) is cut in frontal section, the microfilaments are seen in longitudinal section, suggesting that they are indeed oriented in the long axis of the fold cells. The organization of the microfilaments in the phase ll amniotic fold is similar to that seen in phase l. In frontal sections (Figs. 17C and 18B) or in longitudinal section (Fig. 17D) through the lateral amniotic folds, the apical microfilaments are cut in longitudinal section, again suggesting that filament orientation is in the long axis of the cells. In some sections the filaments are in bundles (Fig. 17C) similar to the "stress fibers" described by Buckley and Porter (1967). Similar bundles are also seen in phase l (Fig. 15C). Sections through the nipple of the delta demonstrate the considerable cytoplasmic blebbing in this region (Fig. 17B). Some microfilaments were located apically below the blebbing region, but they showed no organization comparable to that illustrated by adjacent non-nipple regions (Fig. 17C). The phase lll amniotic orifice possesses a complement of microfilaments circumferentially oriented around the orifice lip (Fig. 19B). The characteristic arrangement of apical filaments, a region of interdigitation, and numerous desmosomes is seen in the amniotic orifice (Fig. 19A). [Since 1977 when Forbes Davidson wrote his dissertation, some additional scanning electron micrographs (SEMs) made in the Jacobson lab were found some 30+ years later. I (AGJ) could not find the negatives, so I scanned the micrographs as they were, including the indications, in my hand writing, indicating where regions were chosen for higher magnification SEMs. These four illustratioms follow.] [The illustration below is a low power (50X magnification) for orientation to the higher power SEMs that follow. They will be amniotic fold (314), amniochorionic ridge (315), and delta with a nipple (310). Figure 20 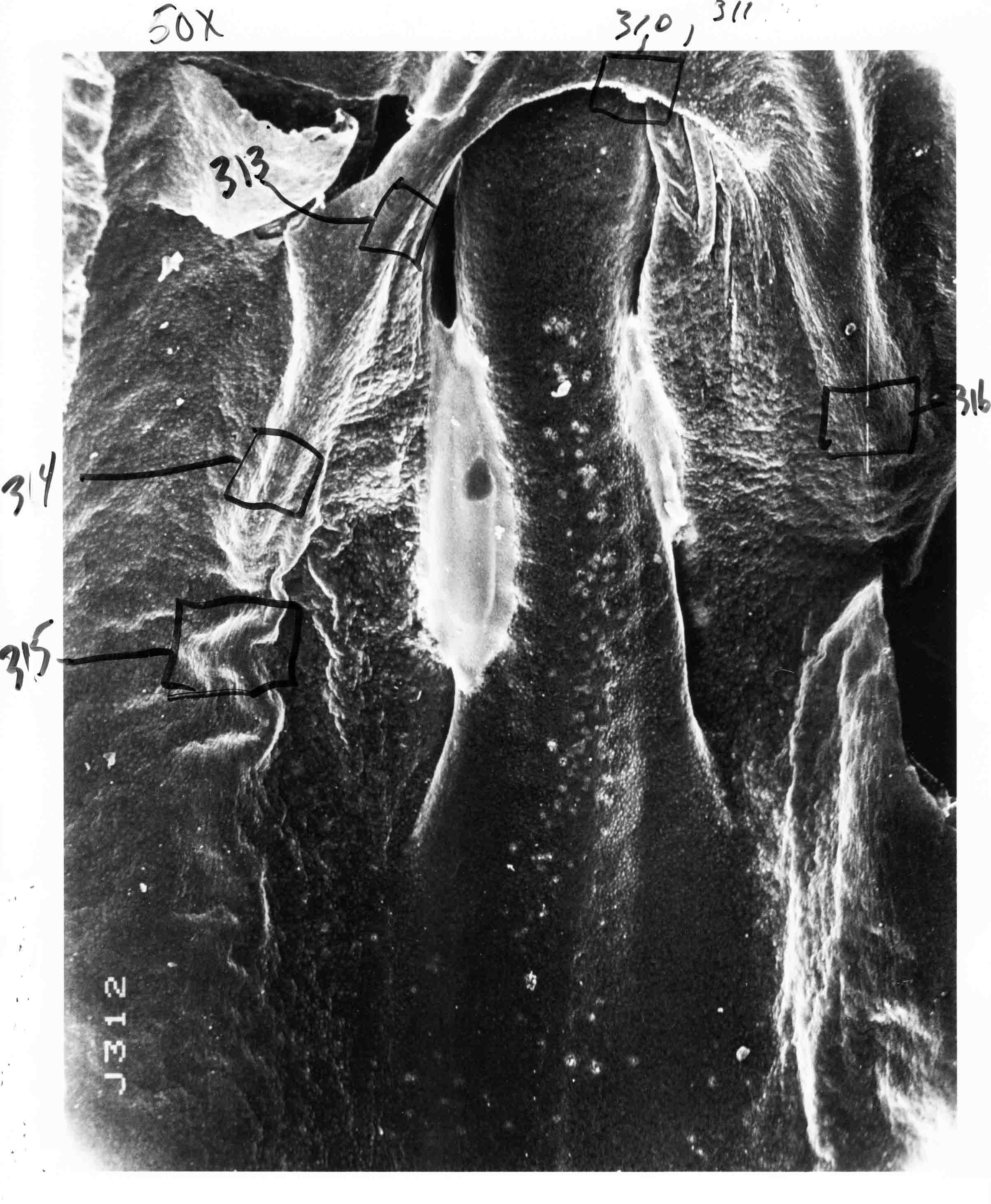 The SEM below is of lateral amniotic fold (314). Figure 21  This SEM shows a portion of a lateral amniotic fold at 1000X magnification. The long amniotic fold cells are flanked by more isodiametric cells of the chorion (left) and amnion (right). The SEM below is a portion of the amniochorionic ridge at 500X magnification. Figure 22  The amniochorionic ridge cells are elongated and occupy a wider swath between chorion and amnion than does the amniotic fold. The SEM below is of the delta and its nipple (310) at 1000X magnification. Figure 23 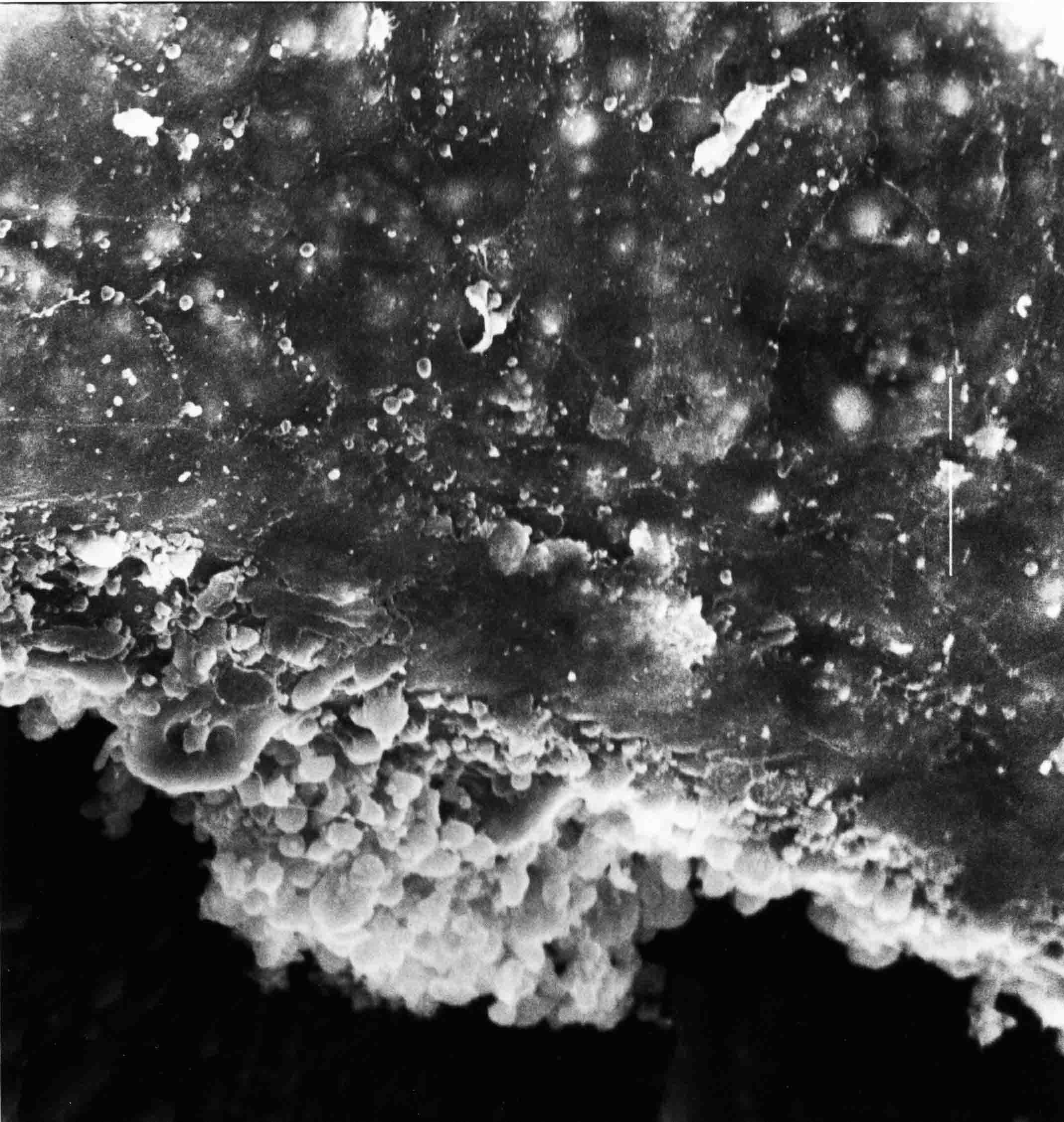 The nipple is covered with many cellular blebs.] Localization and Formation of the Amniochorionic Ridge How is the amniochorionic ridge positioned within the extraembryonic ectoderm; providing sufficient area for the amnion? At least two hypotheses can be invoked to explain the position of the ridge. One, the localization might represent a stress line that is imposed by tissues in other locations in the blastoderm. Two, the localized change in cell shapes could be the result of embryonic induction. Lillie (1903) favored the idea that the lateral amniotic folds were the result of traction (i.e. stress) forces placed upon the somatopleure by the fusing headfold, but that the amniochorionic ridge formed by an unspecified process of self-differentiation. Experiments by Lillie (1903) and Adamstone (1948) demonstrated that continued formation of the lateral amniotic folds was blocked if the headfold was cauterized. Since it is possible that the forming lateral amniotic folds in turn exert a stress or tension on the extraembryonic ectoderm and cause formation of the amniochorionic ridges, I cauterized the headfold in phase l embryos to stop lateral amniotic fold formation. If amniochorionic ridges still formed in such experiments, it would suggest that amniochorionic ridge formation is independent of lateral amniotic fold formation. A series of embryos having either a phase l amniochorionic ridge or an early (i.e. just forming) amniotic headfold were set up in Auerbach cultures and then cauterized in the midline of the amniochorionic ridge or amniotic headfold in such a manner that the midline region was fused to the overlying vitelline membrane. The embryos were then reincubated for varying periods of time. The results are identical whether one cauterized the amniochorionic ridge or the amniotic headfold. This experiment is identical to those of Lillie and Adamstone with the exception that Auerbach cultures were used and could be examined at any time after cauterization. (Neither Lillie nor Adamstone monitored the formation of the amniochorionic ridge, but rather assayed whether or not lateral amnotic folds formed.) Figures. 24A-C illustrate embryos at various intervals after cautery was performed. If the embryos are allowed to develop, there is a continued formation of the amniochorionic ridge such that the ridge extends behind the forming tailbud and forms a large oval amniochorionic ridge extending the entire length of the embryo (Fig. 24A). In comparison to normal embryos, the maximum distance between the two amniochorionic ridges in cauterized embryos is amost equal to that in the normal phase ll/lll embryos. Figure 24 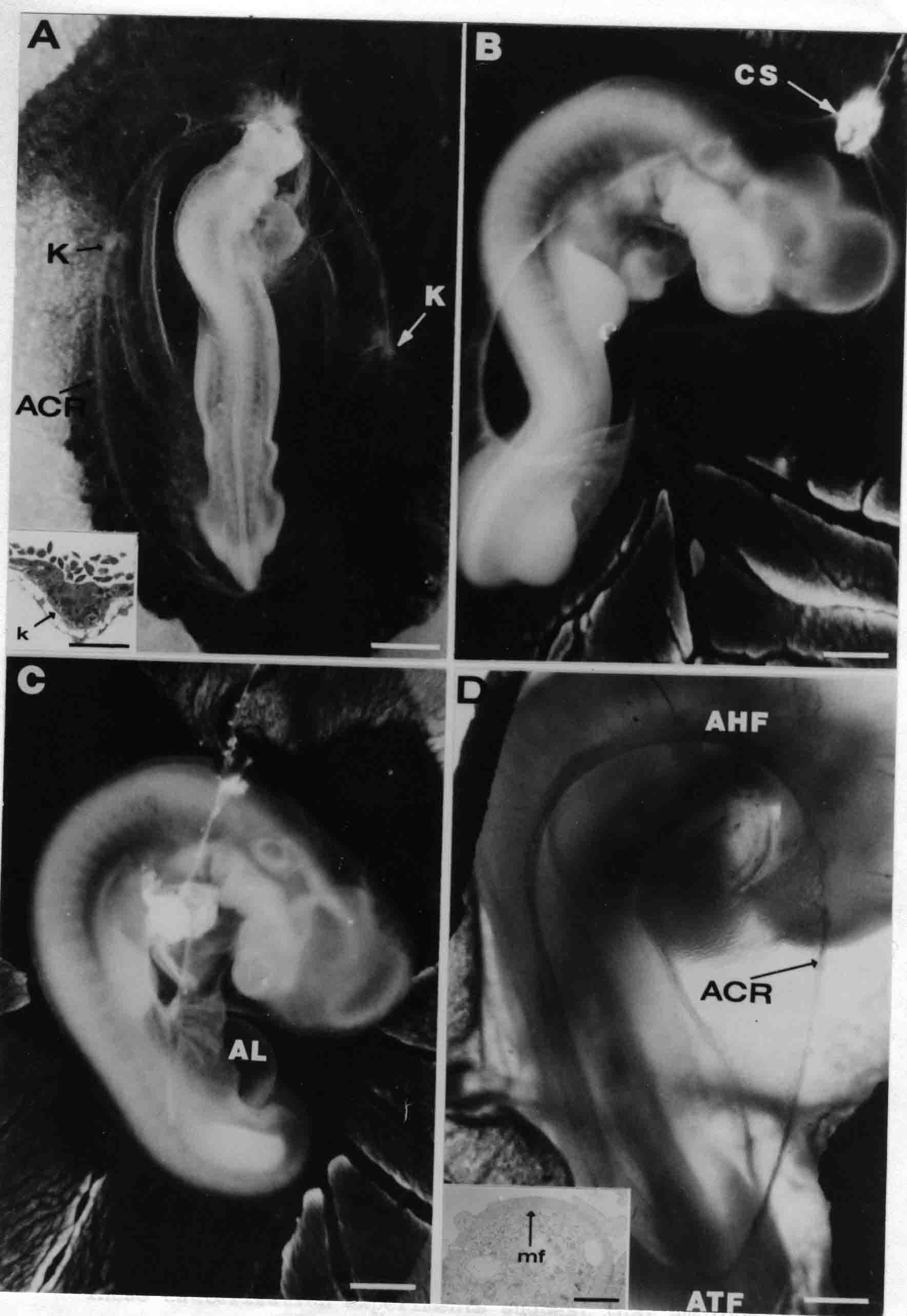 Figure 24 Cauterization of the amniotic headfold. A. Photograph of an embryo 24 hours after the headfold was cauterized. At t zero, the headfold was over the mesencephalon. The oval amniochorionic ridge has formed behind the tailbud. K indicates the knot of cells that occurs in this type of experiment. Scale bar = 1 mm. Inset scale bar = 50 microns. B. An embryo 48 hours after cautery of the headfold. At t zero the headfold was over the prosencephalon. The tail fold has moved anteriorly over the hindlimbs. CS indicates the cautery site. Scale bar = 2 mm. C. An embryo 53 hours cauterization of the amniotic headfold. At t zero the head fold was over the prosencephalon. The tailfold has moved anteriorly almost to the wingbuds so as to form a small amniotic orifice. The allantois (AL) is visible. Scale bar = 2 mm. D. An example of an embryo in which the amniotic headfold movement was found to be blocked in an otherwise normal embryo. The amniotic tailfold (ATF) has moved up over the tailbud and a large amniotic orifice has formed. The amniochorionic ridge is indicated (ACR). Scale bar = 0.5 mm). Inset: a low magnification electron micrograph through the amniochorionic ridge. A zone of what appears to be microfilaments cut in cross section underlies the plasma membrane. Inset scale bar = 2 microns. Once the amniochorionic ridge has extended beyond the tailbud of the embryo, the tail fold of the amnion forms in a manner identical to that seen in normal embryos. However, in cauterized embryos the tailfold moves far anterior as compared to its movement in normal embryos. In several instances the large orifice was observed eventually to close off over the level of the heart. A seroamniotic raphe is formed in those tailfolds that move anteriorly (Fig. 24C) whereas a raphe is never seen in the tailfold of normal embryos. The leading edge of the tailfold is often thickened in the midline (Fig. 24C), but no structure comparable to the nipple seen in phase ll is demonstrable. Occasionally embryos are found (usually during the hot summer months) in which there has apparently been a block in the posterior movement of the headfold (Fig. 24D). Subsequently the amniotic tailfold formed and moved anteriorly to create an unusually large amniotic orifice. The appearance of these naturally occurring embryos does not seem to be significantly different from those that have been cauterized. Together with the fact that amniotic ridges form around the head, and later around the tail, before there are amniotic folds in the areas, these cauterization experiments suggest that amniochorionic ridge formation is not dependent upon stresses supplied by amniotic folds. The question is then, are amniochorionic ridges formed in correct positions by embryonic induction? Occasionally twin embryos have been found in batches of normal embryos and two are shown in Figures 25C and 25D. Figure 25C shows two embryos at approximately right angles to one another. The common amniochorionic ridge is arranged symmetrically with respect to both embryos. Very interestingly, no amniochorionic ridge is found in the region indicated by the asterisk; in a normal embryo, amniochorionic ridge would be found in that region. Figure 25D shows two embryos side by side; the amniotic headfold is over the level of the heart. It seems apparent that whatever the mechanism of localization of the amniochorionic ridge, there must exist some mechanism to ensure that the dimensions of the ridge are proportional to the size of the embryo. Strong evidence for a programmed localization of the amniochorionic ridge as opposed to it representing a stress line is the finding of numerous embryos in which the development of the embryo is highly abnormal yet the amniochorionic ridge appears in it normal position and sometimes forms an amniotic fold. Figure 25A illustrates an acephalic embryo in which the amniochorionic ridge has formed in its normal location. Figure 25B shows another acephalic embryo in which the amniotic headfold has covered a portion of the embryo. Figure 25 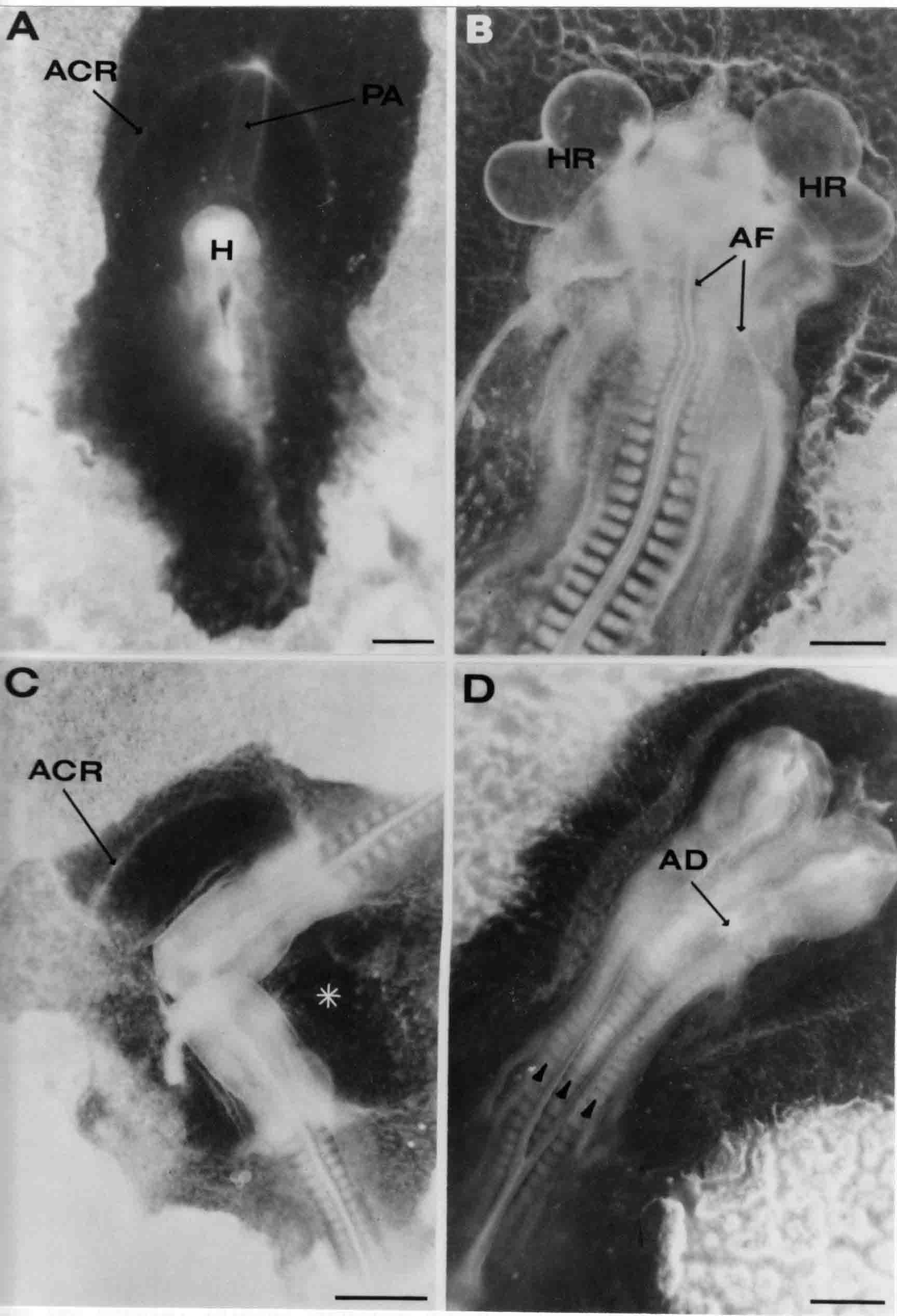 Figure 25 Amniotic fold formation in abnormal embryos. Scale bar = 0.5 mm in all photographs. A. An acephalic embryo in which the development of the head and of the embryo is grossly abnormal. The amniochorionic ridge (ACR) has formed in its normal location. The proamnion (PA) can be seen. B. Another acephalic embryo in which the amniotic fold (AF) has moved posteriorly over the embryonic body. This embryo exhibits cardia bifida: the two heart rudiments (HR) are located to the right and left sides. C. An example of twin embryos at approximately right angles to one another. They have a common amniochorionic ridge (ACR). No amniochorionic ridge appears in the area indicated by the asterisk. D. Side-by-side twin embryos with three rows of somites (arrowheads). The amniotic headfold has moved over the paired heads and an amniotic delta (AD) is visible. Another set of onservations in the context of amniochorionic localization is the very strong correspondence that exists between the most posterior levels of the lateral body fold and amniochorionic ridge. As the amniochorionic ridge differentiates at progressively more posterior levels, it is paralleled by the development of the lateral body folds. This correlation was first noticed in embryos that had been surface-stained since this allows one to see easily both the amniochorionic ridge and the lateral body fold. All embryos examined have shown this relationship and it is also demonstrable in those embryos in which the headfold is delayed with respect to the rest of the embryo (Figs. 25B and 25C). That lateral body fold is not required for amniochorionic ridge formation is indicated by the finding of acephalic embryos having seemingly normally positioned amniochorionic ridges. The relationships between the two events, lateral body fold and amniochorionic ridge formation, is not causal, but rather probably programmed by an event common to both. Movement of the Amniotic Headfold and Amniotic Orifice Closure Several authors (Shore and Pickering, 1889; Ryder, 1886) proposed that cranial flexure and head extension drive the early stages of amniogenesis. They suggest that cranial flexure dips the head while head extension drives the head beneath the ectoderm thereby forming the amniotic headfold. Two observations made in the course of the present study suggest that this hypothesis is untenable. Embryos that had not yet formed the amniotic headfold, but did have an amniochorionic ridge (i.e., early phase l) were prepared as Auerbach cultures and injected beneath with Sandor carbon-agar so that the embryo could be photographed in situ. The embryos were photographed at the beginning and after the headfold had covered part of the head. Photographic prints were made at a constant magnification and the distance measured between the otic vesicle and the tip of the head and between otic vesical and the headfold. During the time interval investigated, the otic vesicle-tip of head distance increased slightly (10.3%) while during the same time there was a 17.3% decrease in the otic vesicle-headfold distance as the headfold moved posteriorly. The finding of numerous acephalic embryos also suggests that headfold formation is not initiated by nor dependent upon cranial flexure since the headless embryos can develop amnions. The posterior movement of the amniotic headfold is generally thought to be the result of level-for-level fusion of the lateral amniotic folds in such manner that the amnion is "zipped up" in an anterior to posterior direction. One prediction of the fusion model is that the putative site of fusion ought to be a transitory cell population, that is the cells at the fusion site are constantly being left behind as fusion occurs at progressively more caudal levels. One way to test whether this site of fusion (the amniotic delta region) is a stable population is to mark it in some manner. In a one series of embryos I marked the delta region with carbon. Results were similar whether the marks were made at phase l, phase ll, or phase lll. In all cases, carbon marks made in the delta region stayed there. If the carbon mark overlapped both delta and anterior to delta, then later carbon was found in both the delta and in the raphe anterior to the delta. In a second marking series, carbon was placed on the right lateral amniotic fold. The usual result of this experiment is that the carbon remains in the delta once it has reached that point. In some embryos, carbon was found later in both the delta and the raphe. This variation probably results from the difficulty of marking only lateral amniotic fold and not adjacent amnion and chorion as well. The first two sets of experiments suggest that the delta is a stable population of cells since carbon particles placed in the delta remain there. The possibility exists, however, that the large mass of cells in the delta traps the particles in a nonspecific manner and the particles remain in place while the cells themselves may move around. In a third set of marking experiments, carbon was placed in the chorion anterior to the delta in the seroamniotic raphe at various distances from the delta to determine whether the carbon would be left behind as the delta moved posteriorly. It appears that if a phase l chorion is marked, the carbon is left behind, whereas if the amniotic folds are in phase ll or lll, the carbon is carried along to some extent, although in all cases the carbon still ends up farther away from the delta than it was at the beginning of the experiment. The total distance moved by the delta is greater than the distance that the carbon is carried. These carbon-marking experiments are summarized in a diagram (Fig. 26). Figure 26 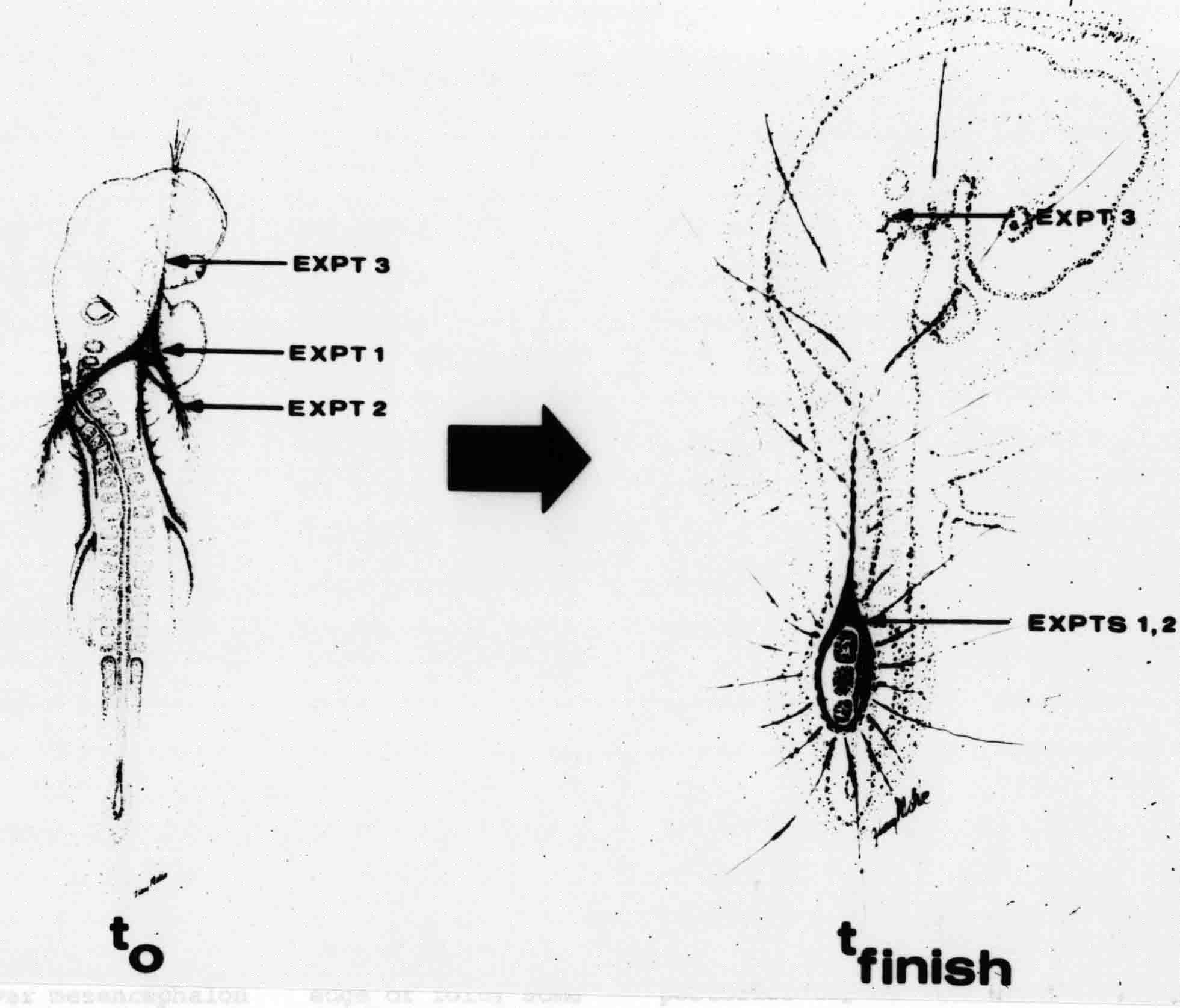 Figure 26 Diagram of the carbon marking experiments. Carbon marks were placed as shown at t-0 (left). Marks were placed in the amniotic delta in experiment 1, in the right lateral amniotic fold in experiment 2, and in the chorion anterior to the headfold at the midline in experiment 3. t-finish (right) shows the position of the carbon marks after a suitable time interval. In expt. 1, the carbon remains in the delta. In expt. 2 the carbon tended to remain in the delta once it reached that location. In expt. 3, the carbon remains behind in the chorion. Tension in the Lateral Amniotic Folds Some simple cutting experiments were performed to examine the nature of the tensions in the amniotic folds. When the amniotic fold is cut with iridectomy scissors, there is an immediate gaping of the cut area that is followed by a curling up of the just-cut ends. Figure 27 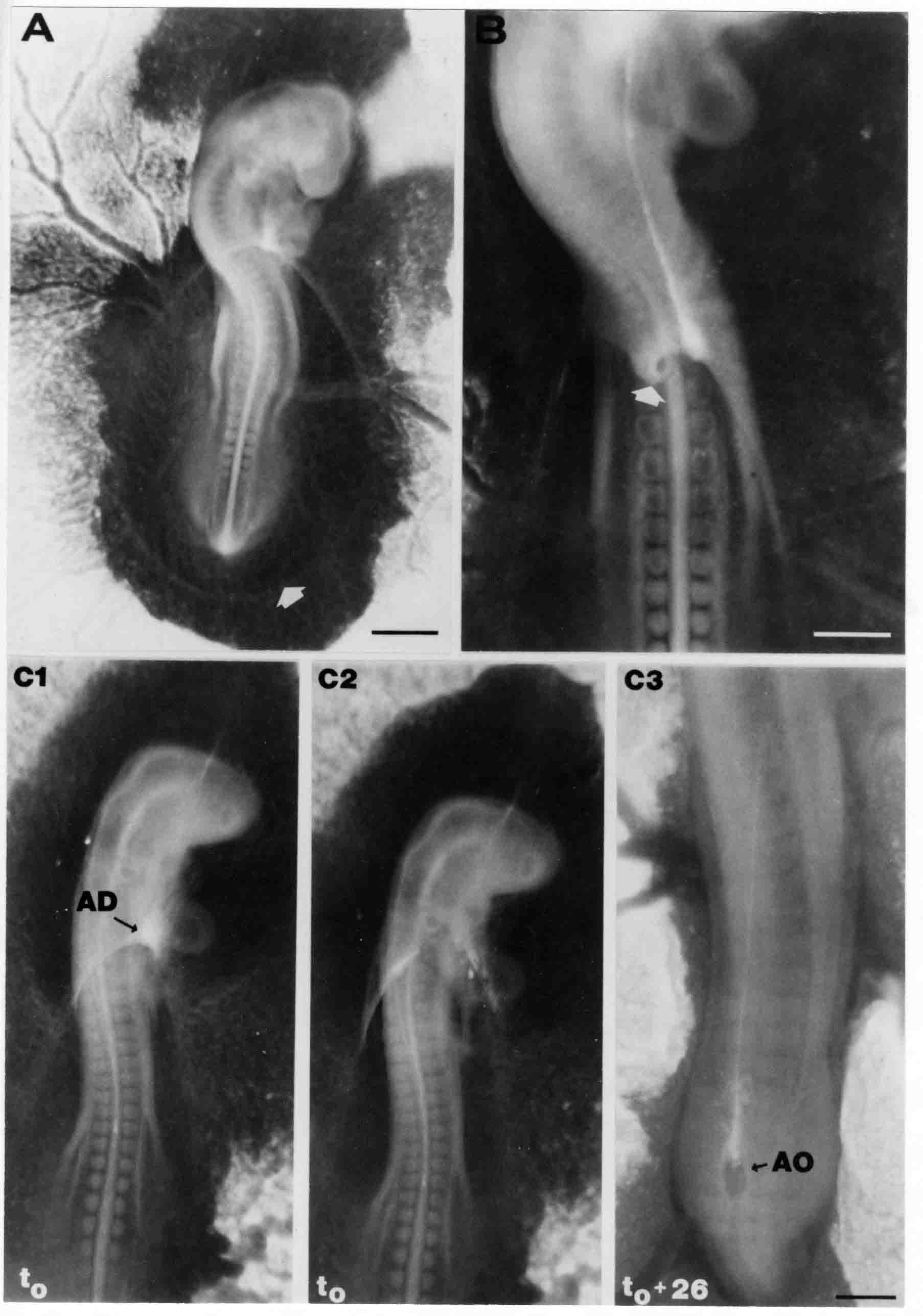 Figure 27 Cutting of the Lateral Amniotic Folds, and Excision of the Amniotic Delta. A. An embryo eighteen hours after both right and left lateral amniotic folds were cut. At t-0, the amniotic headfold wa over the mesencephalon. In the intervening 18 hours, the amniotic fold has reached the posterior tip of the heart ventricle and the amniochorionic ridge (arrow) has formed around the tailbud. Scale bar = 1 mm. B. An embryo immediately after the left lateral amniotic fold was cut with iridectomy scissors. Gaping of the cut occurred immediately and the cut end next to the delta has curled into a C-shape (arrow). Scale bar = 0.5 mm. C1-C3. An example of excision of the amniotic delta. All photographs are at the same magnification. Scale bar = 0/5 mm. C1. Appearance of the embryo before the amniotic delta (AD) was excised. C2. The same embryo as in C1 immediately after the the amniotic delta was excised with iridectomy scissors. C3. The embryo 26 hours later. The amniotic orifice (AO) is almost closed off. Effects of Cytochalasin B on Amniotic Fold Formation. The existence of organized microfilament bundles in the cells of the amniotic folds during all three phases suggested that these microfilaments might be involved in the caudal movement of the amniotic headfold. Since the function of a number of microfilament-mediated systems is disrupted by the drug cytochalasin B (Wessells et al., 1971), this drug was tested for its effects on amniotic fold function. The most effective dosage was found to be 1.0 ml of a 2 X 10 to the minus fifth molar cytochalasin in Tyrode's. Control experiments using an equivalent amount of the carrier DMSO in Tyrode's or Tyrode's alone had no effect. Cytochalasin B was injected between the embryo and the vitelline membrane in almost all cases; in some instances, 0.5 ml was injected beneath the embryo and 0.5 ml on top of the embryo to test its effects on lateral body fold formation. The duration of the treatment varied from 45 minutes to 18 hours. The effects of cytochalasin B were found to be similar at all stages of amniotic fold formation. If a phase l embryo in which the head fold covers the mesencephalon is treated with cytochalasin B, several effects are noted during the ensuing period. The heartbeat slows gradually and becomes more and more arhythmic with time. Flexure and torsion of the head appear to be blocked also. The amniotic headfold, which previously covered the head region, regressed during the period of treatment to a position roughly midway between the head and the area opaca. The general outline of the regressed amniotic fold closely resembles that of the amniochorionic ridge during phase l. The amniochorionic ridges extend to the level of somites 12-14. The total length of the amniochorionic ridge is greater than the combined length of amniotic folds plus amniochorionic ridge that existed at the beginning of the experiment. The maximum distance between the amniochorionic ridges in these cytochalasin B-treated embryos is on the average greater than that of normal phase l embryos (3.45 mm versus 2.25 mm)(Fig. 28A). During phase ll, the effects of cyrochalasin B treatment are similar to those seen in phase l. The apex of the amniotic fold moves to a more anterior level and the lateral amniotic folds move more laterally after treatment (Fig. 28B). In addition to its effect on the phase ll amniotic fold, cytochalasin B also causes the regression of the lateral body folds. This effect is often quite variable if the drug is applied only to the top of the embryo; if a portion of the cytochalasin B is injected underneath the embryo, relaxation of the body folds is readily invoked (Figs. 29C-1-2). (Cytochalasin B is not normally injected beneath the embryo as it can much more readily cause heart stoppage.) Cytochalasin B appears to have little effect on the amniochorionic ridge and does not cause the disappearance of the ridge in those embryos in which the amniotic headfold and lateral bodyfold have regressed (Figs. 28C and 29D). Treatment with cytochalasin B is the only instance so far in which there has been noted a separation of the most posterior levels of the amniochorionic ridge and lateral body folds. The response of the phase lll amniotic orifice to cytochalasin B resembles that in phases l and ll. When orifices of varying circumference are treated, they respond with an increase in the size of the orifice (Figs. 28D and 29B-1-2). The tail fold of large to moderate sized orifices is caused to relax in a fashion similar to that in the cytochalasin-treated headfold (Fig. 28A) so that after treatment the tailfold is found behind the tailbud as the amniochorionic ridge. The lateral margins of the orifice move farther laterally and the headfold moves to a more anterior level as the orifice expands. In some cases there has been noticed a tearing of the raphe anterior to the delta (Fig. 28D); the edge of the apex does not tear, however, and maintains its integrity, suggesting that the effect of the cytochalasin B is not to rip open (i.e., unfuse) the raphe beginning at the delta. Cytochalasin B causes a rapid relaxation or regression of the amniotic folds. It was rather surprising to find that older embryos left overnight after treatment often showed a reversal of the cytochalasin B effects; in one series of embryos the reversal began three hours after treatment (Figs. 29B, 1-3). Reversal has not been seen in phase l embryos and may reflect the generally more sensitive nature of these younger embryos. Figure 28 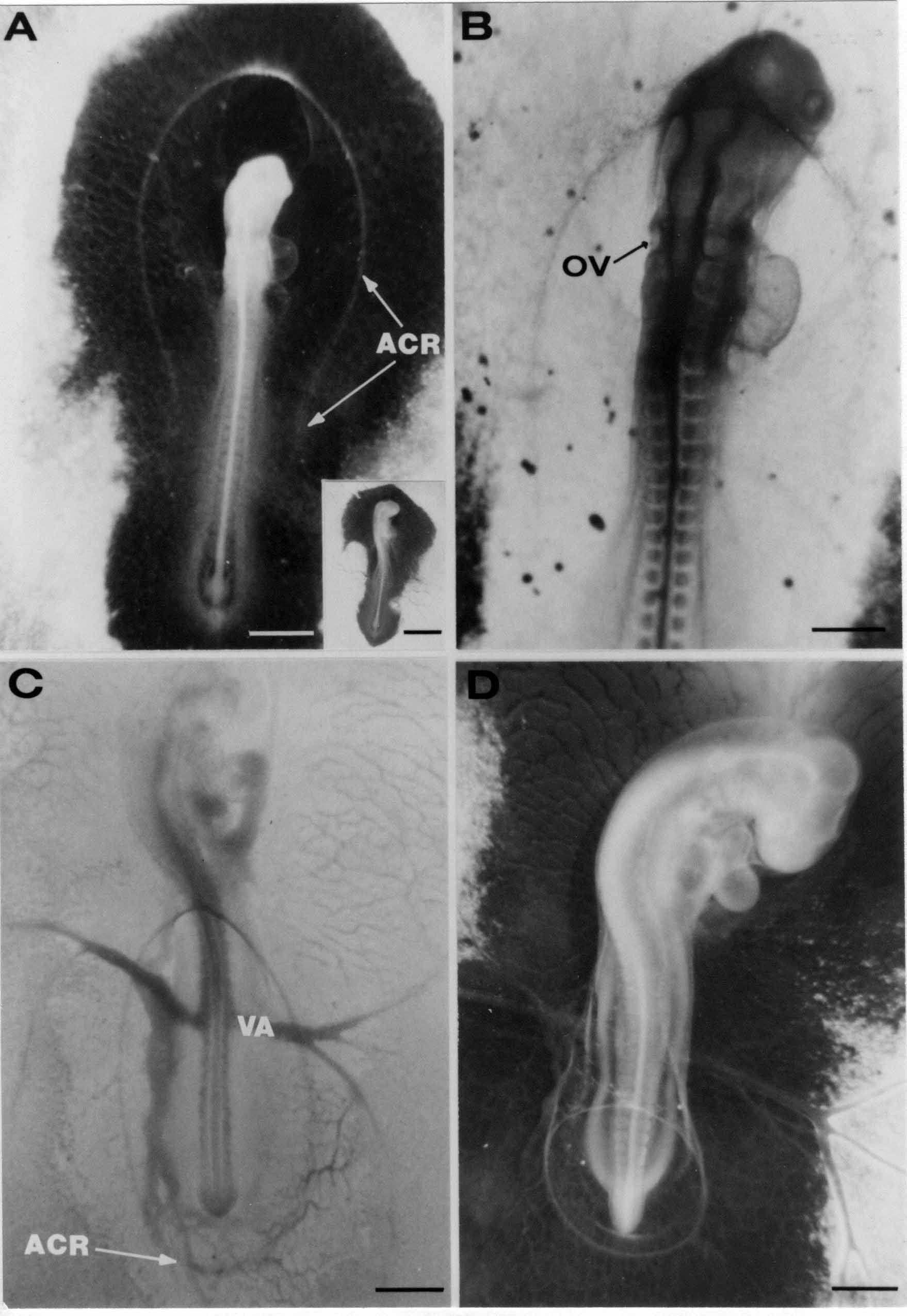 Effects of Cytochalasin B on Amniotic Folds. A. An embryo seven hours after treatment with cytochalasin B (CB). At time zero, the amniotic headfold was over the mesencephalon. Seven hours after treatment with CB, the amniotic fold has relaxed so that only an amniochorionic ridge (ACR) is found in the area pellucida. The ACR extends a considerable distance posteriorly. Very little shaping of the head has occurred. Scale bar = 1 mm. Inset: Control embryo of the same age as the treated one. Compare the head shape and amniotic fold position with the CB-treated embryo. Scale bar = 2 mm. B. An embryo after 80 minutes treatment with 0.5 ml of 2 X 10 to the minus five molar cytochalasin B. The amniotic fold was over the otic vesicle (OV) at start of the experiment; treatmment with CB has caused the relaxation of the amniotic fold so that it noe overlies the mesencephalon. Scale bar = 0.5 mm. C. An embryo 2.5 hours after treatment with 1 ml of 1 X 10 to the minus five molar cytochalasin B. When the experiment began, the amniotic fold was at the level of the vitelline artery (VA). After teatment, the amniotic headfold has regressed anteriorly approximately seven somites. The amniochorionic ridge (ACR) extends behind the tailbud. Scale bar = 1 mm. D. A phase lll embryo 2.75 hours after treatment with 1 ml of cytochalasin B. Treatment has caused the amniotic orifice to increase in circumference 3.85 times. Note that although ripping has occurred in the sroamniotic raphe, the lip of the orifice is a complete circle. Scale bar = 1 mm. Figure 29 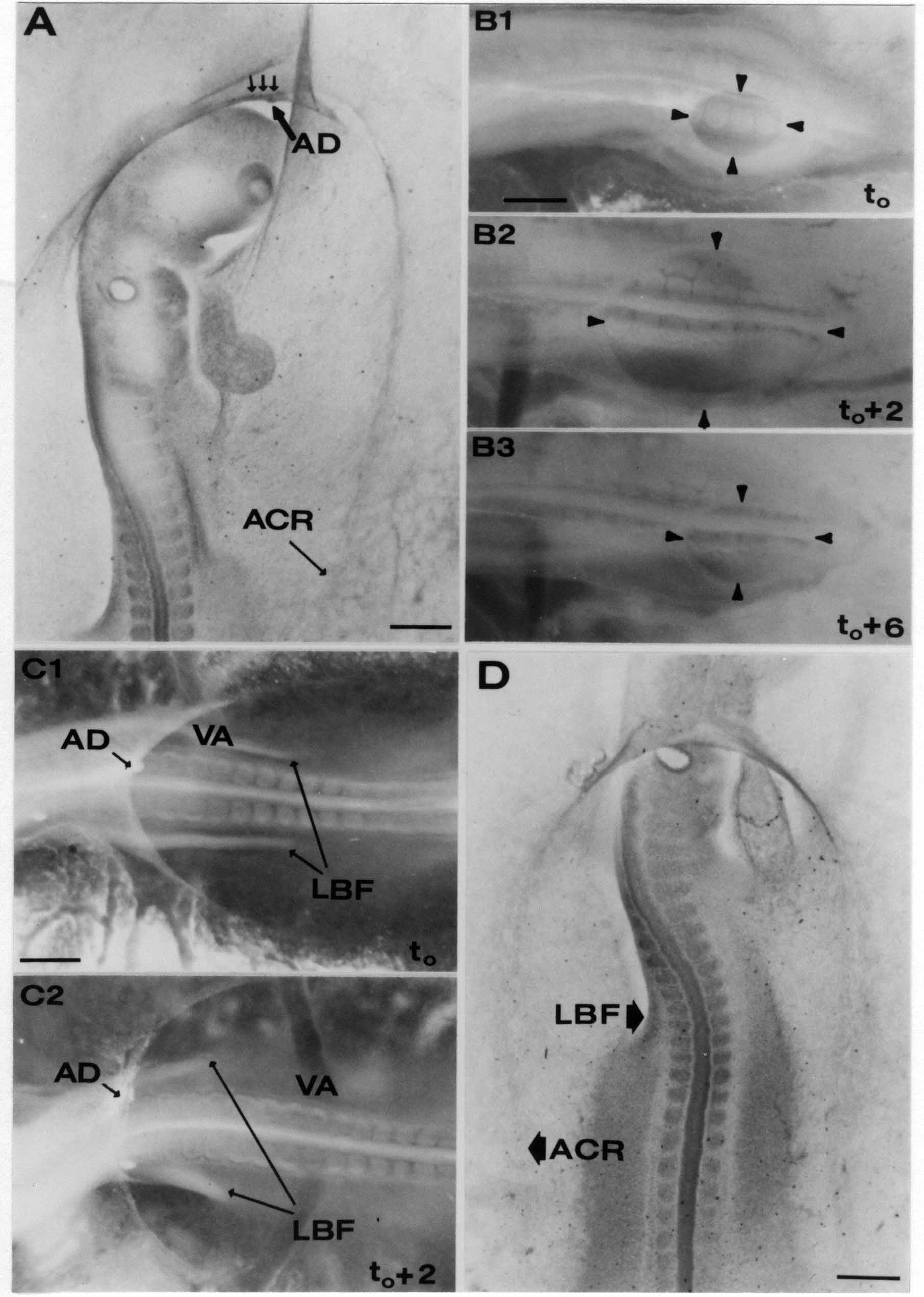 Cytochalasin B Effects on Amniotic Fold and Lateral Body Fold. Scalr bar = 0.5 mm in all photographs. A. Combined carbon marking and cytochalasin B treatment. When the experiment began, the amniotic fold was at the level of the tip of the heart ventricle; after 2.25 hours treatment, the amniotic delta (AD) and carbon marks in the delta (arrows) had relaxed to the tip of the head. The amniochorionic ridge is seen posteriorly (ACR). Since the carbon marks that were placed in the delta have remained there, the relaxation of the headfold of the amnion does not entail an unfusing of the cells of the delta. B1-B3. An example of cytochalasin B-induced amniotic orifice expansion followed by orifice contraction. All photographs are at the same magnification. B1. At t zero, the amniotic orificoe was treated with cytochalasin B. B2. Two hours later, the circumference of the amniotic orifice has expanded 2.25 times. B3. Six hours after the experiment began, the amniotic orifice has begun to contract and the circumference is 1.37 times as great as in B1. C1 and C2. Effect of cytochalasin B on lateral body folds and amniotic folds. Both photographs are at the same magnification. C1. At the beginning of the experiment, the lateral body folds (LBF) are well past the level of the vitelline arteries, and the amniotic delta is about two somites anterior to the vitelline arteries. The embryo is treated with 1 ml of cytochalasin B, 0.5 ml above the embryo and 0.5 ml beneath the embryo. C2. Two hours later, the amniotic delta (AD) is 8 somites anterior to the vitelline artery and the bodyfolds are about 4 somites anterior to the vitelline artery. D. An embryo surface stained with methylene blue to demonstrate the regression of the lateral body folds (LBF), but not of the amniochorionic ridge after treatment with 1 ml of cytochalasin B. The amniochorionic ridge extends much farther posteriorly after cytochalasin B treatment than does the lateral bodyfold. At the beginning of the experiment, the headfold of the amnion was at the level of the second somite. Duration of treatment was 110 minutes. Some Additional Findings. An examination of data on amniotic orifice expansion following treatment with cytochalasin B was of interest. The relative increase in orifice circumference after cytochalasin B treatment is much greater, proportionally, in small initial orifices compared to large initial orifices. A number of smooth muscle drugs were tested for effects on amniotic orifice closure. These drugs were acetylcholine, oxytocin, epinephrine, norepinephrine, and potassium ions. None of these drugs significantly altered amniotic orifice closure, nor affected its time course. [ Dr. Davidson's dissertation presented 26 pages of discussion that focused the many findings of his descriptive and experimental studies and related them to previous studies by others. He began by noting] "... the amniochorionic ridge (the immediate precursor of the amniotic folds) is extremely difficult to visualize in the intact embryo due to lack of contrast between the embryo and the underlying yolk. In this regard it is interesting that the Hamburger and Hamilton (1951) staged series of chick development does not show the amniochorionic ridge." He states further "The best rendition of the amniotic folds and amniochorionic ridge .. has been obtained by the method developed in this study, that is, by staining the dorsal surface of the embryo." Selected "quotes" and my [comments or summaries] follow: "Two major events are involved in the formation of the amnion. The first is the development of the amniochorionic ridge. The second is the posterior movement of the amniotic headfold and the closure of the amniotic orifice." "The amniochorionic ridge occupies a fairly precise and consistent location in the area pellucida between the body of the embryo and the area opaca. Measurements show the maximum width between the opposite limbs of the ridge during phases l, ll, and lll is fairly constant. The ridge is first seen at 9 somites (stage 10-) and has an antero-posterior sequence of development, indicating both a chronological and topological localization." "The amniochorionic ridge is a swath of elongated cells arrayed initially as an arc around the head of the embryo and later along the sides of the embryo and behind the tailbud. The amniochorionic ridge raises several questions, including: what determines where the ridge is formed, how does the cellular polarity and alignment of the cells arise, and what function(s) does the ridge serve?" [The amniochorionic ridge (ACR) might be positioned by stress lines in the ectoderm produced by contraction of the amniotic fold cells. Since the ACR that first forms around the head preceeds any amniotic fold formation that is most unlikely. There are other possible sources of stresses in the ectoderm, for example, head elongation and torsion. This is negated by ACR formation in normal sites in headless embryos. The active expansion of the ectoderm as the blastoderm covers the yolk mass should produce stress directed toward the edges of the blastoderm. These stresses could help account for the tautness of the chorion or presumptive chorion. Elongation of the embryo, which is accompanied by elongation of the area pellucida could produce stress in the ectoderm. Other stresses are also known, and how and if the resultant strains in the ectoderm could result in the line occupied by the ACR is not yet known. The ACR comes to occupy its normal site in a number of abnormal embryos. The probability that the ACR is induced in its normal position is likely. The head ACR appears before the head region ectoderm is underlain by mesoderm of the somatopleure so mesoderm is not a likely inductor tissue. The head ACR directly overlies the endoderm. It is a likely candidate for induction vertically. There is also the possibility that planar induction arising from the embryo could occur, but normally sited ACR appears in headless embryos so planar induction from the embryo should not be required. Whatever the cause(s), the ACR consistently appears in its normal site.] [The important question is how do the cells of the ACR become elongated? This swath of ACR cells lies between isodiametric cells of chorion to one side and amnion to the other side. The cells of the ACR might elongate by tensions from contracting amniotic fold cells, but that is not so since head ACR forms before there is any amniotic fold. It seems more likely that ACR cells elongate themselves. The elongation of ACR cells is along the line that will later become the amniotic fold.] "Electron micrographs of the amniochorionic ridge (Figs. 14A and 14B) demonstrate a few microfilaments located deep in the cytoplasm. They are neither abundant nor organized as in amniotic fold. Cytochalasin B has little effect on the stability of amniochorionic ridge." [ACR cells lack the apical surface features seen in the amniotic fold cells that the ridge cells become. Amniotic fold cells have extensive subplasmalemmal bundles of microfilaments, as well as some deeper bundles, that run along the long axis of the cells. The microfilament bundles attach to and end at junctional complexes between cells. Desmosomes are present. ACR cells show none of these apical features.] [Cells of the ACR have a somewhat mesenchymal appearance. As such they may elongate themselves by crawling movements that stretch themselves along the axis of the future amniotic fold. Alternatively, they might elongate by fusion of adjacent cells into a cell with many nuclei as in some vertebrate skeletal muscle cells. That would be quite odd, but I (AGJ) have re-examined the data in the dissertation and what else I could find and cannot eliminate that possibility. In any event, ACR cells are already elongate when they form the apical features of the amniotic fold cells that they become.] [The amniotic fold cells have the enigmatic feature of an apical "pursestring" stretched considerably along the axis of the fold. This puts them in a situation in which contraction of their stretched microfilament "pursestrings" would exert considerable tension along the line of the amniotic fold. Cytochalasin B causes these fold cells to relax the tension produced by their contraction.] [Contraction of the amniotic fold cells may produce all the tension that is needed to move the amniotic head fold posteriorly (and the amniotic tail fold anteriorly). It seems especially clear that tension along the line of the fold is responsible for closure of the amniotic orifice. The experiments of Davidson showing the effect of cytochalasin B on the relaxation of the amniotic orifice, and the later re-contraction of the orifice fully support this interpretation.] [It is quite possible that when these apically located microfilament bundles contract fully, the cell may curl up. Blebs are often seen among these cells as in Figures 21 and 22. The closer to the anterior midline in the head fold, the more blebs are seen. This suggests that there may be progression in the numbers of folded cells in the contracted state the closer to the anterior (or posterior for tail fold) midline the cells are. Once an amniotic fold cell is formed, it moves progressively toward the delta, along with chorion and amnion cells to which it is attached.] [As the amniotic folds are shortened, they may exert tension rather locally on attached adjacent chorion and amnion cells that in turn may divide as many stretched cells do. Division may be delayed somewhat so there would remain elastic stresses imposed on these amnion and chorion cells. These elastic forces may help pull the amniotic folds back into relaxed positions when the tension in the fold is relaxed by treatment with cytochalasin B.] [The changing shape and size of the amniotic folds may be matched by chorion and amnion growth as a result of growth being stimulated by tension from the folds.] [As the amniotic fold cells shorten, they move toward and to the midline of the headfold of the amnion. Attached chorion and amnion cells are also brought to the midline. As the head fold of the amnion continues to move posteriorly, what is to become of the three cell types now meeting at the midline? Chorion cells fuse with chorion cells from the other side, and amnion cells with amnion cells. Contracted amniotic fold cells have no place to go, pile up in the delta, and die. The chorion cells fuse, but some are in excess and they linger in the raphe. Something like that may also apply to the meeting amnion cells. The notion that fusion drives amnion formation (like zipping up a zipper) seems most unlikely, and would need most peculiar mechanics.] The End |