|
Years ago, the genetics group in our department at U. Texas kept a large collection of Drosophila species and studied the biology and evolution of the genus. Jan Durr (later Kinsey, then Grebe) was one of my first Ph.D. students. She decided to work on a problem of Drosophila hybrid lethality. Her work introduced Drosophila into our laboratory. Patterson and Griffen (1944) crossed D. montana females with D. texana males. Only male offspring were produced, suggesting that some incompatibility between D. montana ooplasm and D. texana X-chromosome killed the female hybrid embryos before hatching. Jan set out to determine the embryological nature of this incompatibility. The two species are effectively sexually isolated because of behavioral differences and poor viability of D. texana sperm in the ducts of D. montana females. Hybrid crosses were very infrequent. To get enough hybrid embryos to study, Jan had to resort to interspecific ovary transplantation. That is, she transplanted D. montana ovaries into D. texana female larvae. (J.D. Kinsey 1966 Interspecific ovary transplantation in Drosophila. Transplantation 4:509-512). Jan examined time-lapse movies of development of normal D. montana eggs and of eggs of the lethal hybrid. In syncytial blastema stages of normal D. montana eggs, nuclei are gathering into the peripheral cytoplasm. When contraction occurred in the yolk mass, the nuclei in the peripheral cytoplasm moved toward the poles, and when the yolk mass relaxed, peripheral cytoplasm and nuclei moved away from the poles. In eggs of the lethal hybrid, the movements were more vigorous and the movements in the yolk mass and the peripheral cytoplasm were less coordinated so that the end result was a displacement of peripheral nuclei out of orderly arrangement near the surface of the whole egg and their clustering in bunches. These cells then did not differentiate. When cell wall formation occurred around these displaced nuclear masses, some cells contained more than one nucleus, some nuclei appeared to be polyploid, and some cells contained pycnotic masses. The incompatibility of the D. texana X-chromosome and the D. montana ooplasma was thus expressed at these early stages. Jan's figures 2 and 3 illustrate this (from: J.D. Kinsey 1967 Studies on an embryonic lethal hybrid in Drosophila. J. Embryol. Exp. Morph. 17:403-423). 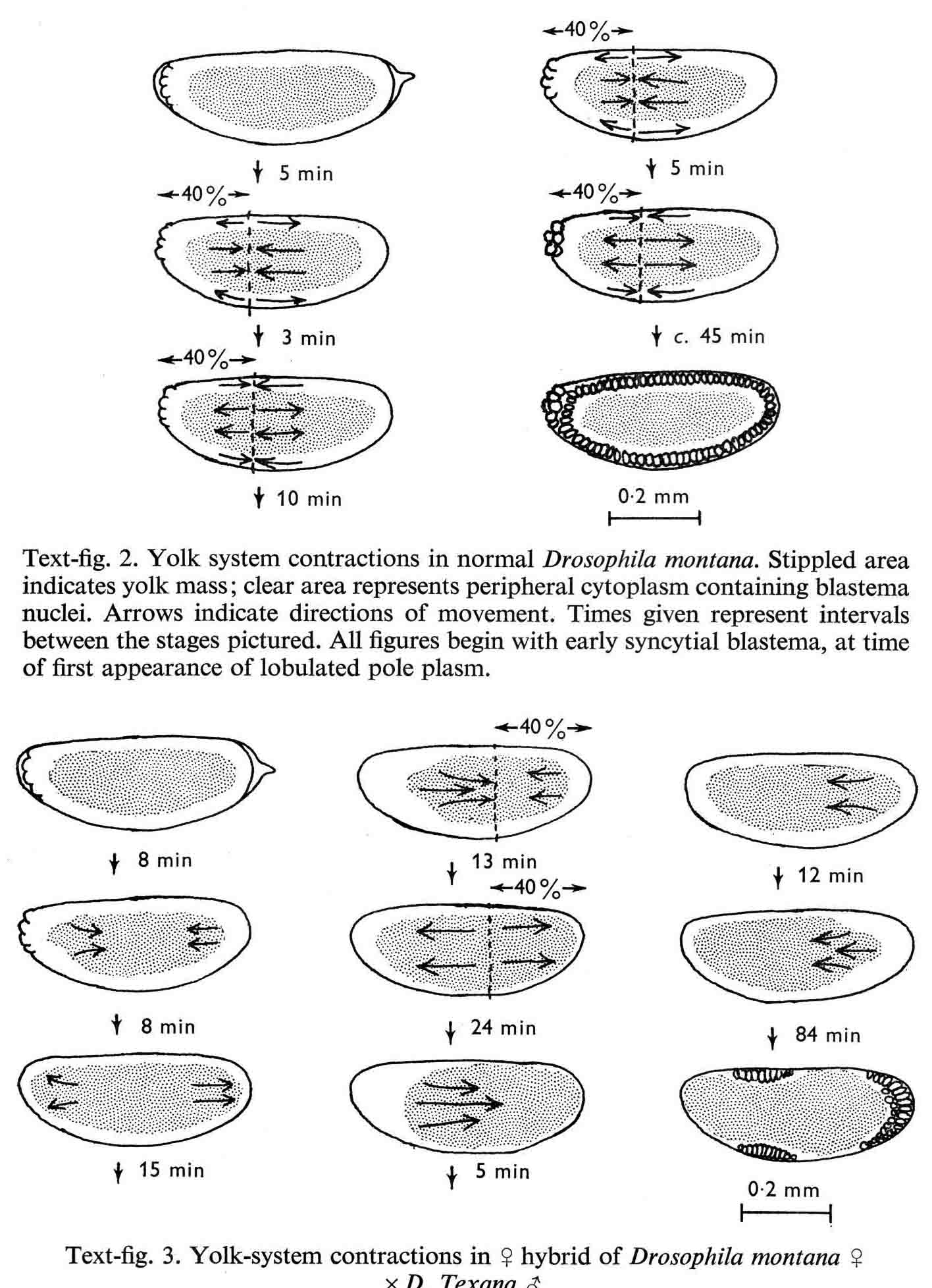 We continued to use D. montana for further studies. This species does not retain its eggs within the ducts as does D. melanogaster. Experiments timed from fertilization are more readily done with D. montana. James C. Wilson, for his Master's degree, made a thorough study of the syncytial stages of the D. montana egg. As had others before, he used time-lapse movies to follow the movements within the egg during these stages. (James C. Wilson 1970 Analysis of Pre-Blastoderm Cytoplasmic and Nuclear Movements in Drosophila montana. Thesis for the degree of Master of Arts, The University of Texas at Austin, August, 1970.) Previous authors using time-lapse movies described a series of "spasms" in syncytial eggs that coincided with nuclear divisions. They used a framing rate of two or four frames per minute. When viewed at 16 frames per second, these framing rates greatly speeded the movements, so they appeared as spasms rather than detailed movements. Wilson used framing rates of 8 or 16 frames per minute. He made 20 movies from which he chose six of the best for detailed analysis. He used two movies each at high magnification of the anterior and the posterior halves of the eggs, and two movies of whole eggs at lower magnification. (A) in the drawing below is a freshly laid egg just after fertilization. The egg is surrounded by a non-cellular vitelline membrane with a protrusion, the micropyle (MI) at the anterior end, through which the sperm entered. Yolk laden cytoplasm (Y) is surrounded by a clearer peripheral cytoplasm (P). This appearance lasts about thirty minutes after the egg is laid. During this time, oocyte maturation divisions produce the female pronucleus and three polar body nuclei. About thirty minutes after the egg is laid, a concentration of yolk laden cytoplasm begins toward a point 40-50 percent of the distance along the long axis from the anterior end. This initial yolk concentration slowly continues for 20 to 30 minutes (B to C). The egg begins (B) to pull away from the vitelline membrane (VM). As the yolk concentrates and the egg get smaller, the periplasm is moved toward the poles (B). At the end of this concentration period, fluid-filled spaces (S) have formed between the anterior and posterior ends of the egg and the vitelline membrane. Some of these early events were described by Imaizumi (1954). During this initial yolk concentration, the male and female pronuclei move away from the peripheral surface into the yolky mass and begin to align close to one another in preparation for fusion and the first nuclear cleavage. 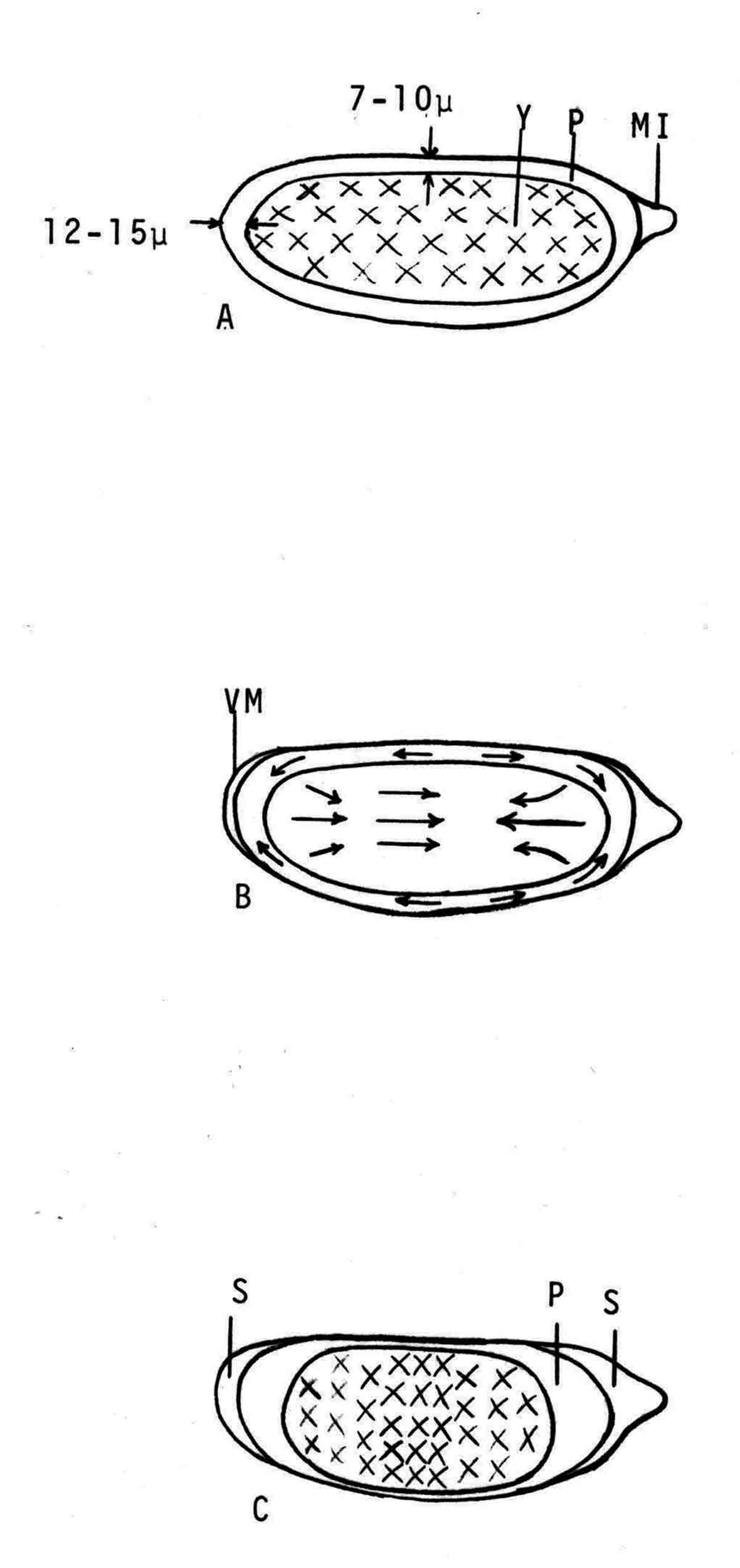 Within a few minutes after initial yolk concentration (shown above, from Wilson's thesis) is complete, the series of 12 movements of peripheral and yolky cytoplasm begin that correlate with the synchronous nuclear divisions within the syncytial egg. The initial yolk concentration (IYC) and the 12 movements are shown in the graph below (from Wilson's thesis). The solid line is the yolk mass length, and the movements are described as expansions and contractions of this parameter. The thickness of periplasm at the poles varies inversely with yolk mass expansion. T.E.L = Total Egg Length. 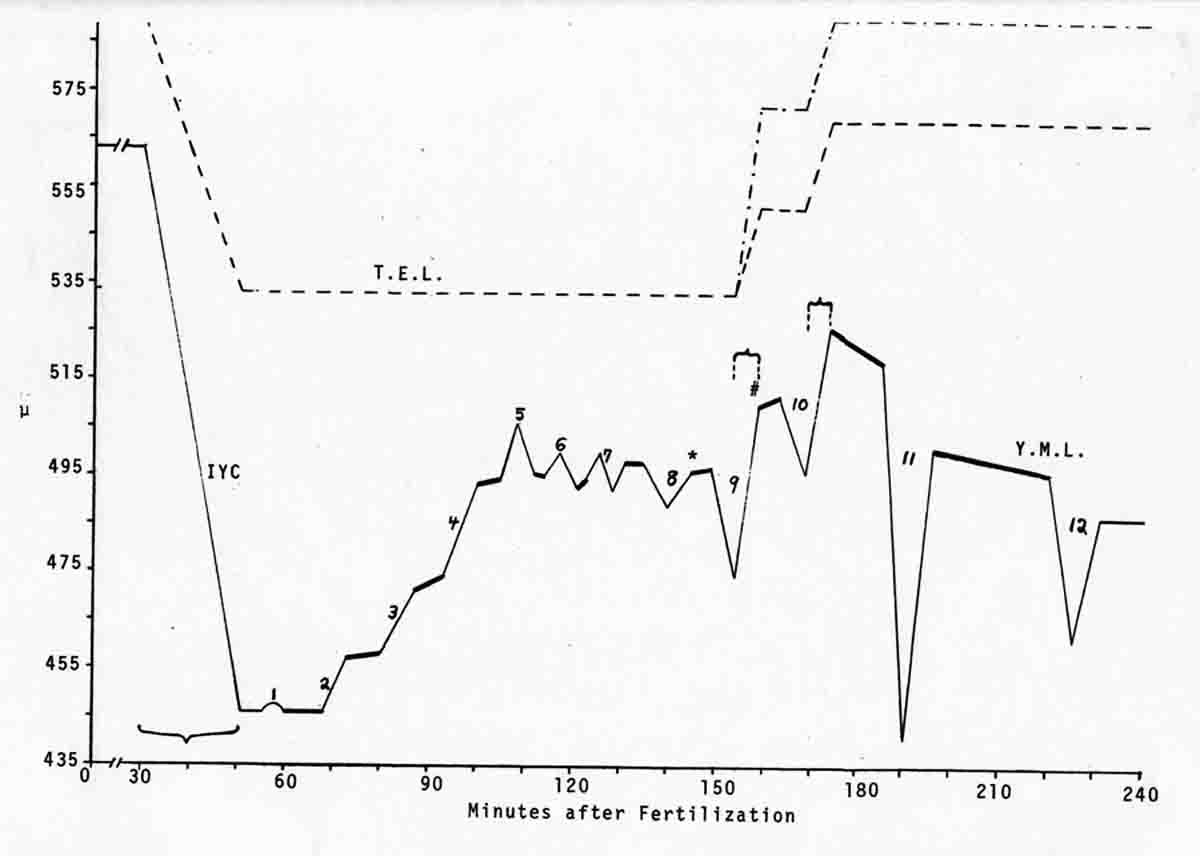 At the end of movement 8, nuclei have reached the poles (*). During the expansion phase of movement 9, (#) pole cells pinch off from the posterior pole of the egg and occupy the former posterior pole space. During the expansion phase of movement 10, the whole egg expands to fill the anterior space within the vitelline membrane. During movements 11 and 12, the total yolk mass length decreases as all parts of the periplasm get thicker. Shortly after movement 12, the peripheral nuclei increase volume and length as they become enveloped in cell membranes. The thousands of peripheral nuclei become cellularized simultaneously, and morphogenetic movements begin almost immediately. The first four movements are shown in the following drawings (from Wilson's thesis). 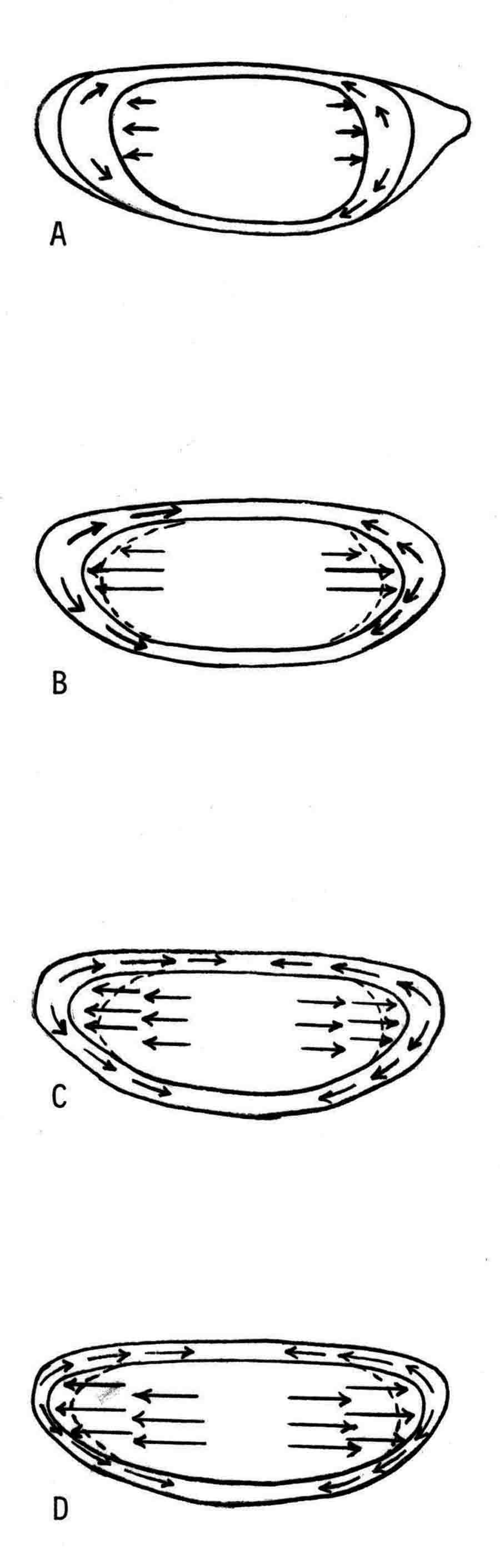 The first movement is almost unnoticeable (only one nucleus is dividing). There is slight movement of some yolk spheres in an expanding direction, and polar periplasm moves slightly near the poles. Movements 2, 3, and 4 are expanding movements, each greater than the movement before. Periplasm depth changes with each of these movements. By the end of the fourth movement (D), periplasm is more evenly distributed around the yolk mass. Movements 5 and 6 consist of an expansion followed immediately by a contraction, so there is litte or no change in yolk mass length. The graph shows movement 7 consists of expansion-contraction-expansion followed by movement 8 with contraction-expansion. In other eggs, movement 7 is expansion-contraction followed by expansion-contraction-expansion in movement 8. By the end of movement 8, nuclei have reached periplasm of both poles. By the end of movement 9, which is a strong contraction-expansion, the pole cells are extruded at the posterior pole where they occupy the posterior space between the egg and the vitelline membrane. By the end of movement 9, nuclei occupy the entire periplasm, but are not closely packed. This begins the blastema stage. Movements 10, 11, and 12 are also very strong contraction-expansion movements. The expansion is usually less than the contraction phase. (Some of these last three movements were the ones Kinsey described.) The drawings below (from Wilson) show a movement such as in movements 10, 11, and 12. During these movements the periplasm becomes thicker with each movement, and it is a time when almost all nuclei still in the yolk mass move into the periplasm. (PC = Pole Cells) 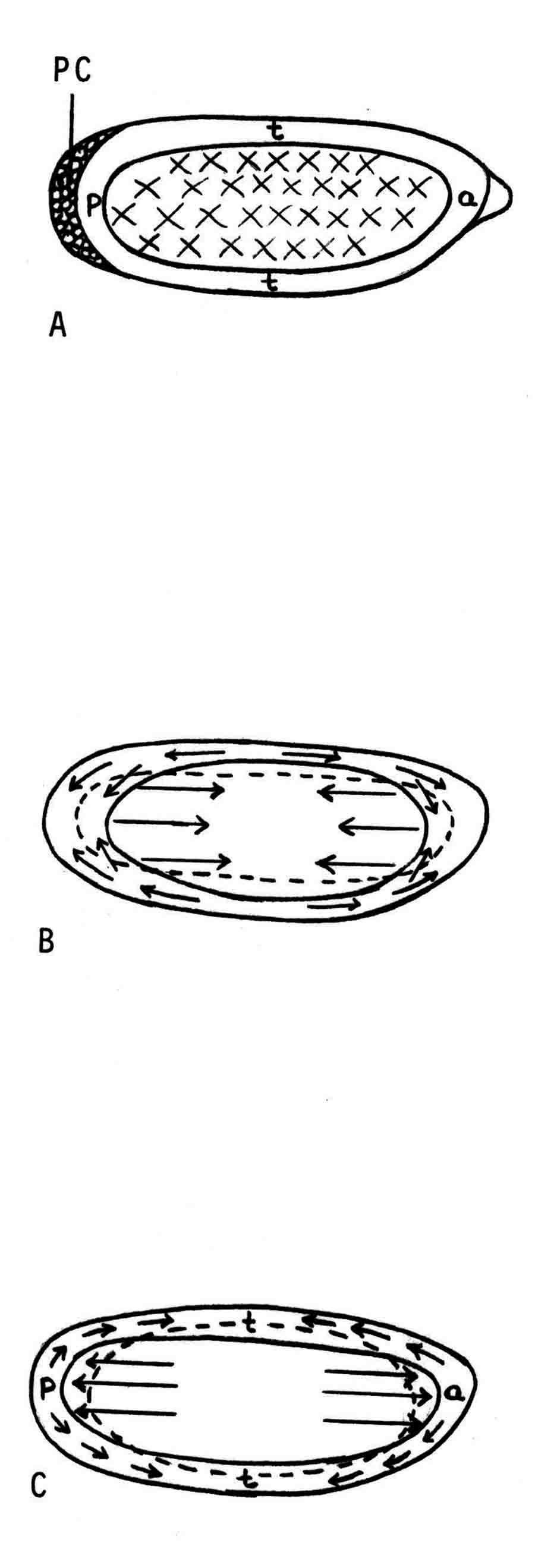 The several D. montana embryos examined all showed the same 12 movements. Others have suggested that there are 13 movements in D. melanogaster: M. Zalokar and I. Erk, (1976) Division and migration of nuclei during early embryogenesis of Drosophila melanogaster. J. Microsc. Biol. Cell 25:97-106) Perhaps the two species differ in number of movements and divisions, or perhaps the movements have been counted differently. At the end of movement 12, there are thousands of nuclei in the periplasm and the simultaneous encapsulation of the nuclei within cell membrane begins. This process is described by S.L.Fullilove and A.G. Jacobson, 1971, Nuclear elongation and cytokinesis in Drosophila montana. Developmental Biology 26:560-577. There are about 3500 spherical nuclei in the peripheral cytoplasm of the egg after the twelth movement and nuclear division. The electron micrograph (EM) of a cross section of the egg at this time (below) shows the egg membrane raised in folded hillocks over each spherical nucleus with flattened areas of membrane between the nuclei (N). The cleavage furrows begin in the flattened regions. 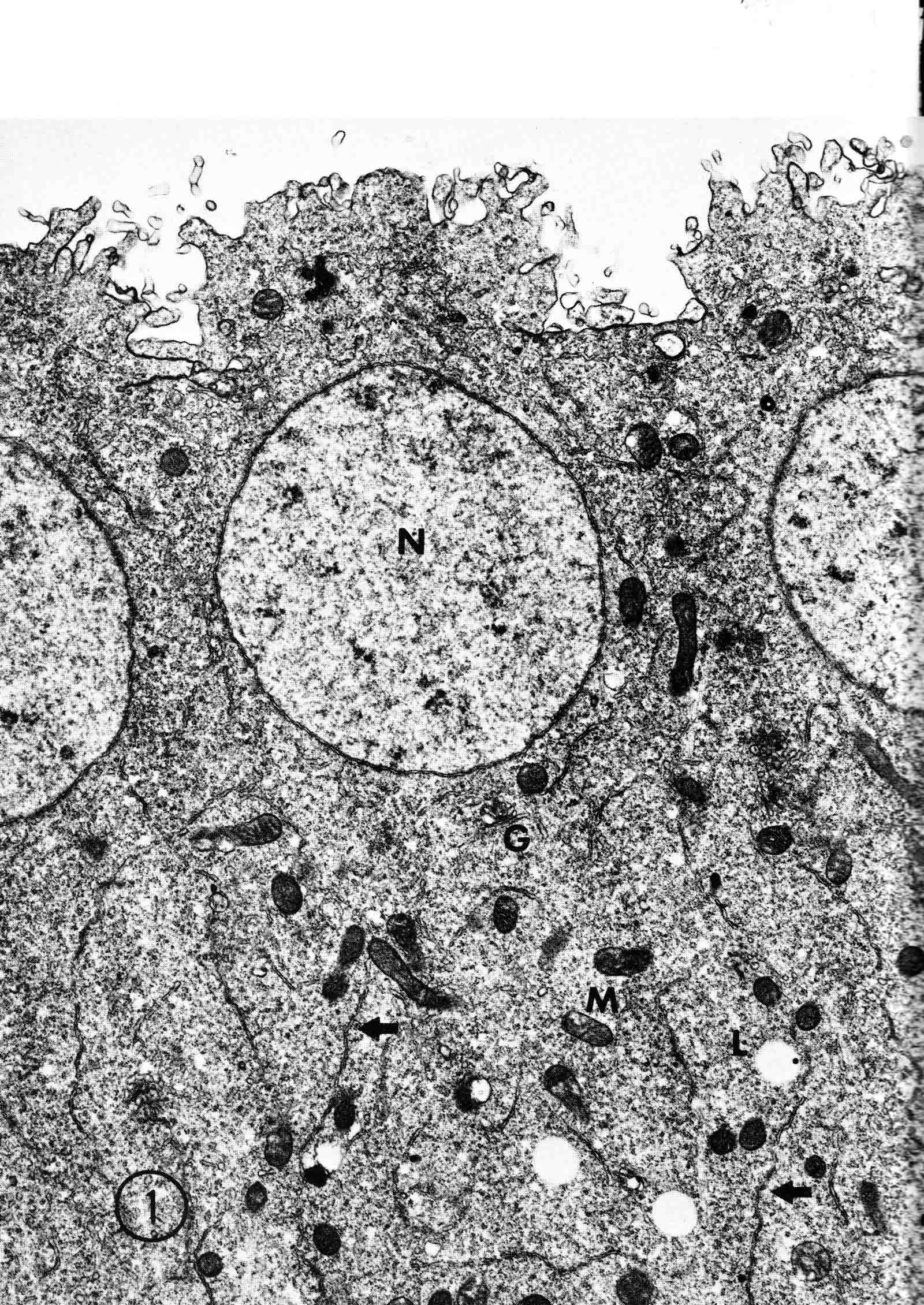 Shortly after the twelth movement, the peripheral nuclei begin to simultaneously elongate, they increase their volume, and cleavage furrows commence to extend inward from the flat cell surfaces between the nuclei. The EM below shows a cross section of an egg when the elongation is maximal (from Fulliove and Jacobson, 1971). 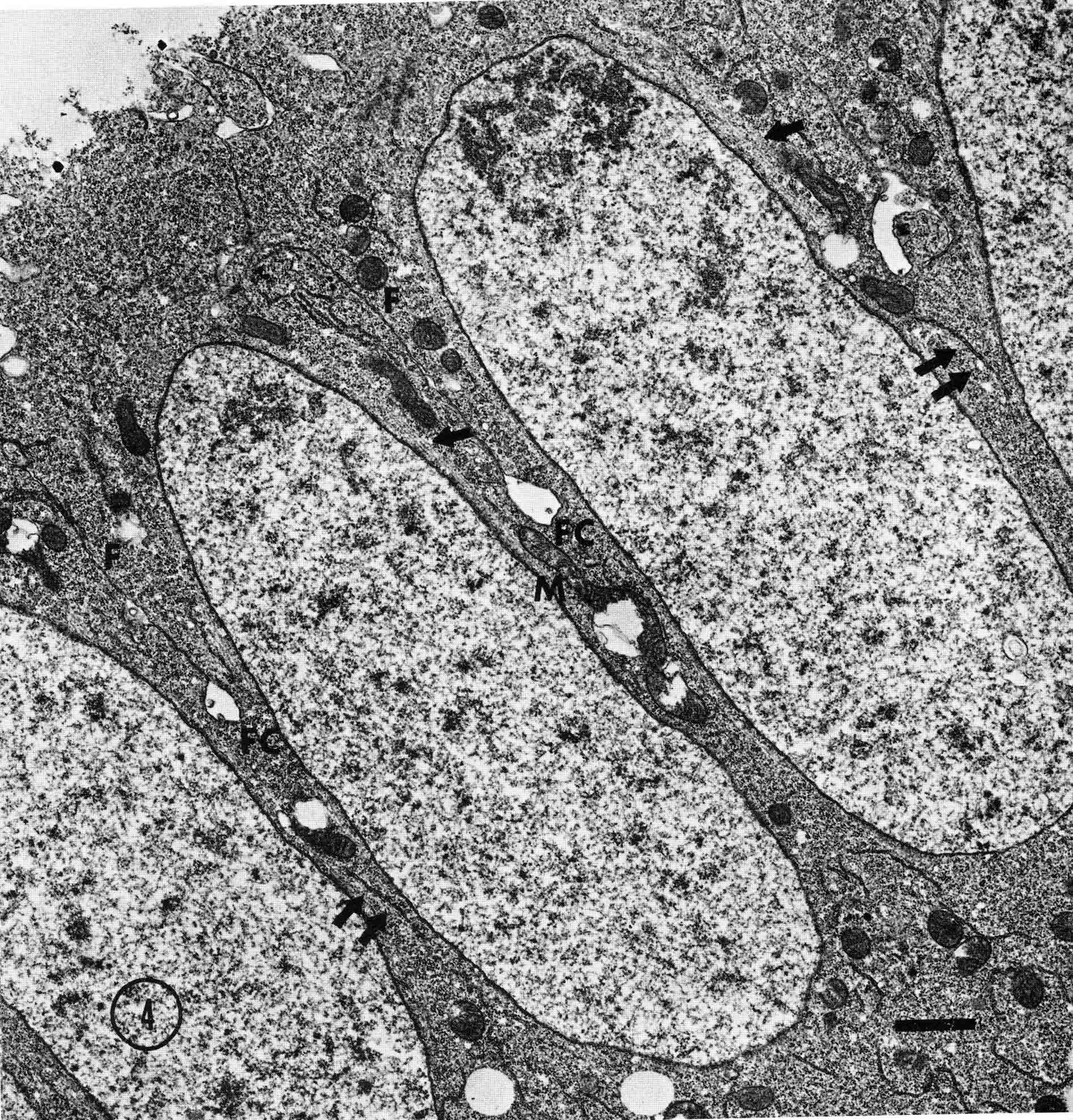 The EM above is at the same magnification (bar = 1 micron) as the EM of the spherical nuclei. During nuclei elongation, which takes 35 to 40 minutes, the nuclear volume increase 2.4 fold. The cleavage furrows in the EM (F) have reached about a third of the way down the length of the elongated nuclei, and the advancing base of the cleavage furrows form a furrow canal (FC). Arrows indicate bundles of microtubules that run parallel to the nuclei. Microtubules (arrows) in association with the nuclei are shown at a higher magnification in the EM below (bar = 1 micron). 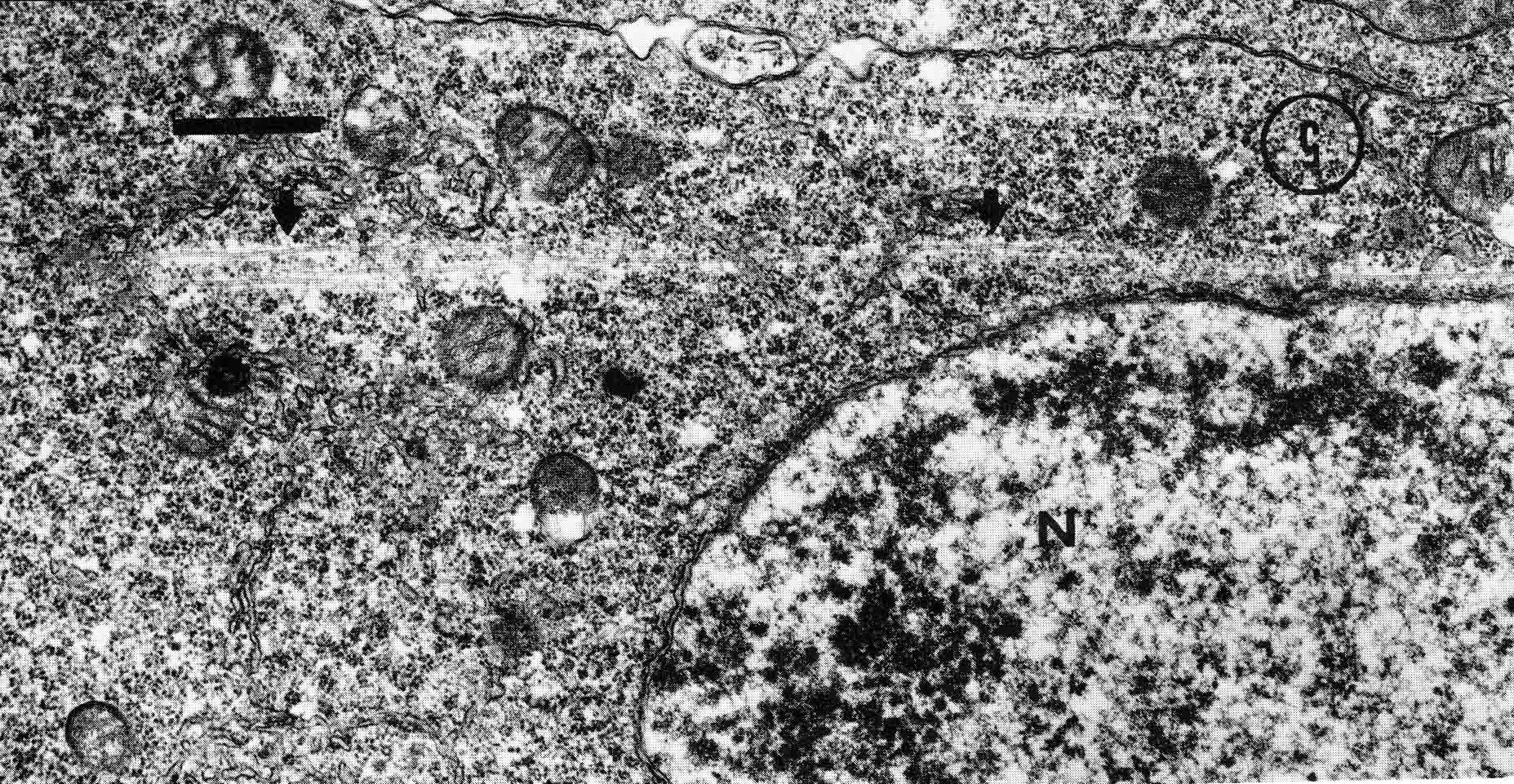 Our attempt to illustrate a three dimensional view is in the following drawing. The cleavage furrows are a continuous hexagonal array around the whole egg. We suggest that contraction of a continuous array of microfibrils in the continuous cleavage furrows could draw the cleavage furrows deeper into the egg. 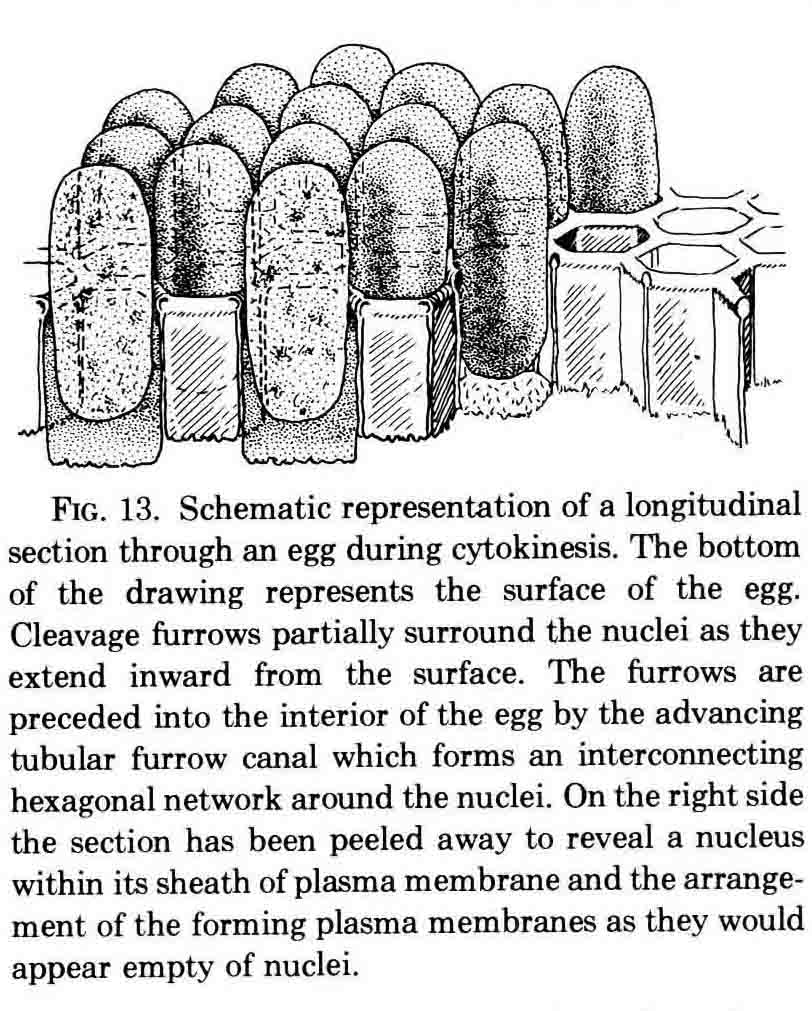 The drawing below summarizes the processes of nuclear elongation and cytokinesis during formation of the cellular blastoderm. 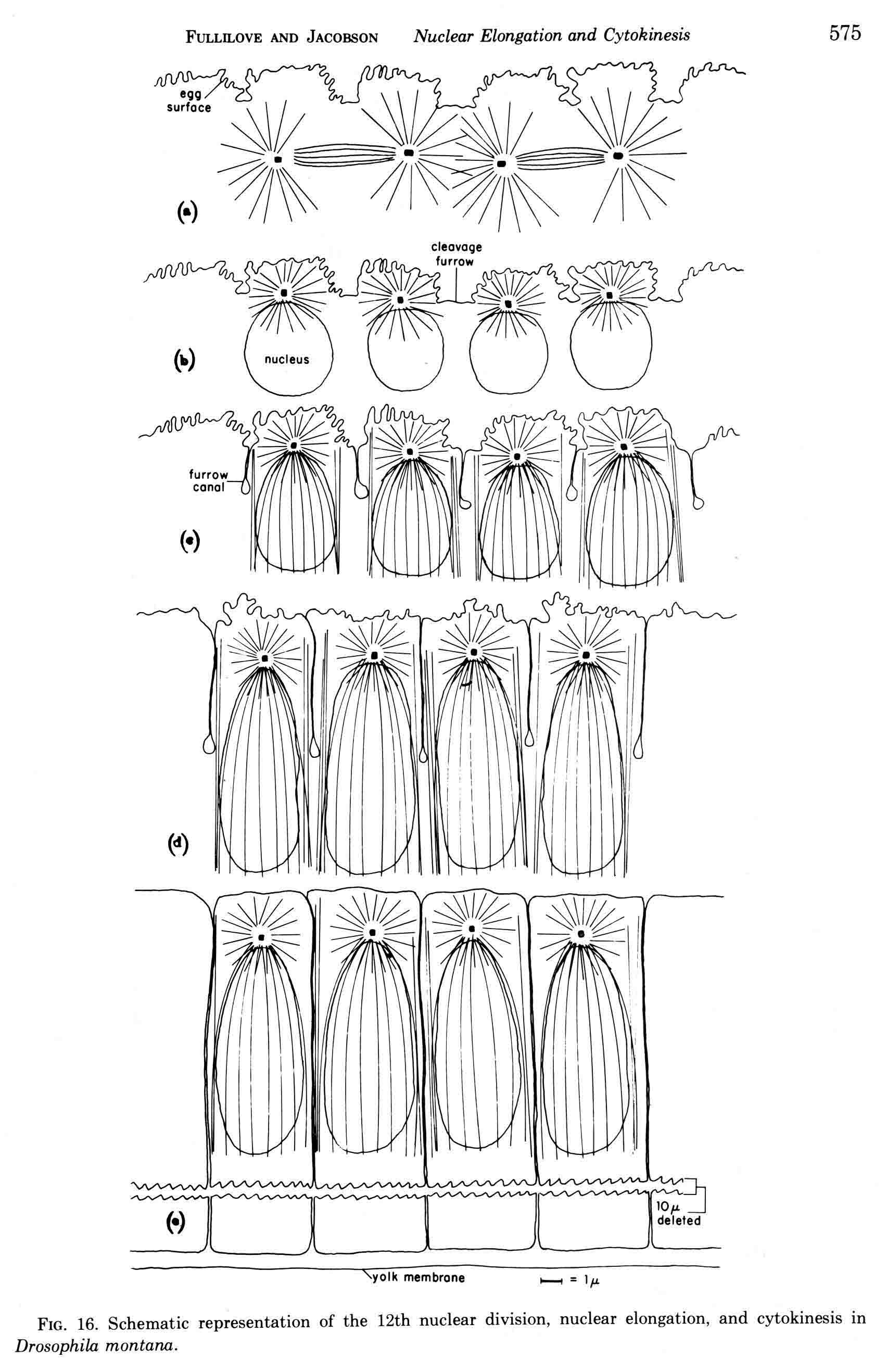 The drawing is to scale and emphasizes the 2.4 increase in volume of each nucleus. One must question the source and nature of local materials that go into the increased mass of each nucleus. We calculated that about 2.3 X 10 to the sixth square microns of new membrane would be needed to coat the 3500 new cells. Our time lapse movies showed that cleavage furrow progress was slow until the base of the nuclei as reached, then occurred faster thereafter until complete when it reached the yolk mass. We estimated that sufficient egg-surface membrane was present in the convoluted membrane above each spherical nucleus to be drawn into the forming furrows to account for the cleavage furrow membrane added in the slow phase of furrow progress. By the time the slow phase is complete, the surface above each nucleus is flat. During the fast phase, from the base of the elongated nuclei to the yolk mass, a second source is paired sheets of membrane within the cell that move about in our movies, and are oriented parallel to the cleavage furrow axis of growth. Another question raised by this cellularization process is how are the positions of cleavage furrow initiation established. We argue that the methods of position initiation proposed by Rappaport (1965,1969) are sufficient. That is, by interactions between pairs of asters and the cell surface. Asters from daughter nuclei would place furrows between daughter nuclei. Also, in the crowded nucleus population of the syncitial blastoderm, neighboring asters of non-daughter nuclei should interact with cell surface and thus inition sites would surround each nucleus. Susan L. Fullilove and I have written a chapter on Drosophila embryology. It is Chapter 21. Embryonic Development: Descriptive, by Susan L. Fullilove and Antone G. Jacobson, with scanning electron micrographs by F.R. Turner. In: The Genetics and Biology of Drosophila, Volume 2c, Edited by M. Ashburner and T.R.F. Wright. Academic Press. London, New York, San Francisco. 1978. Drosophila Embryology |