|
Two postdocs, Bruce Lipton and David Packard, began studies of somite developent in our lab. Bruce first analysed normal somite development in the chick embryo (1), then made an experimental analysis of the mechanisms of somite morphogenesis (2). The descriptive analysis used light and electron microscopy and time-lapse cinematography. Most of the study focused on the formation of the first six somites. These early somites are formed somewhat differently from later ones. To avoid frequently seen artifacts, embryos were fixed before removal from the yolk. The egg is opened into a bowl and the embryo positioned upward. A glass ring, 15mm in diameter, is placed to make a well around the embryo. The fixitive, 2% buffered glutaraldehyde, is put into the well and also injected into the yolk underlying the embryo. After 15 minutes, the glutardehyde is removed and replaced with osmium tetraoxide. After a time, the embryos are excised from the yolk and resuspended in fresh osmium for a total period of 45 minutes, then rinsed in 1% NaCl, dehydrated in graded alcohols and propylene oxide then embedded in plastic. Sections are cut on an ultramicrotome, thick (1 micrometer) for light microscopy, and thin sections for electron microscopy. The differences between this technique and ordinary fixation and paraffin sections are illustrated below (from 1). 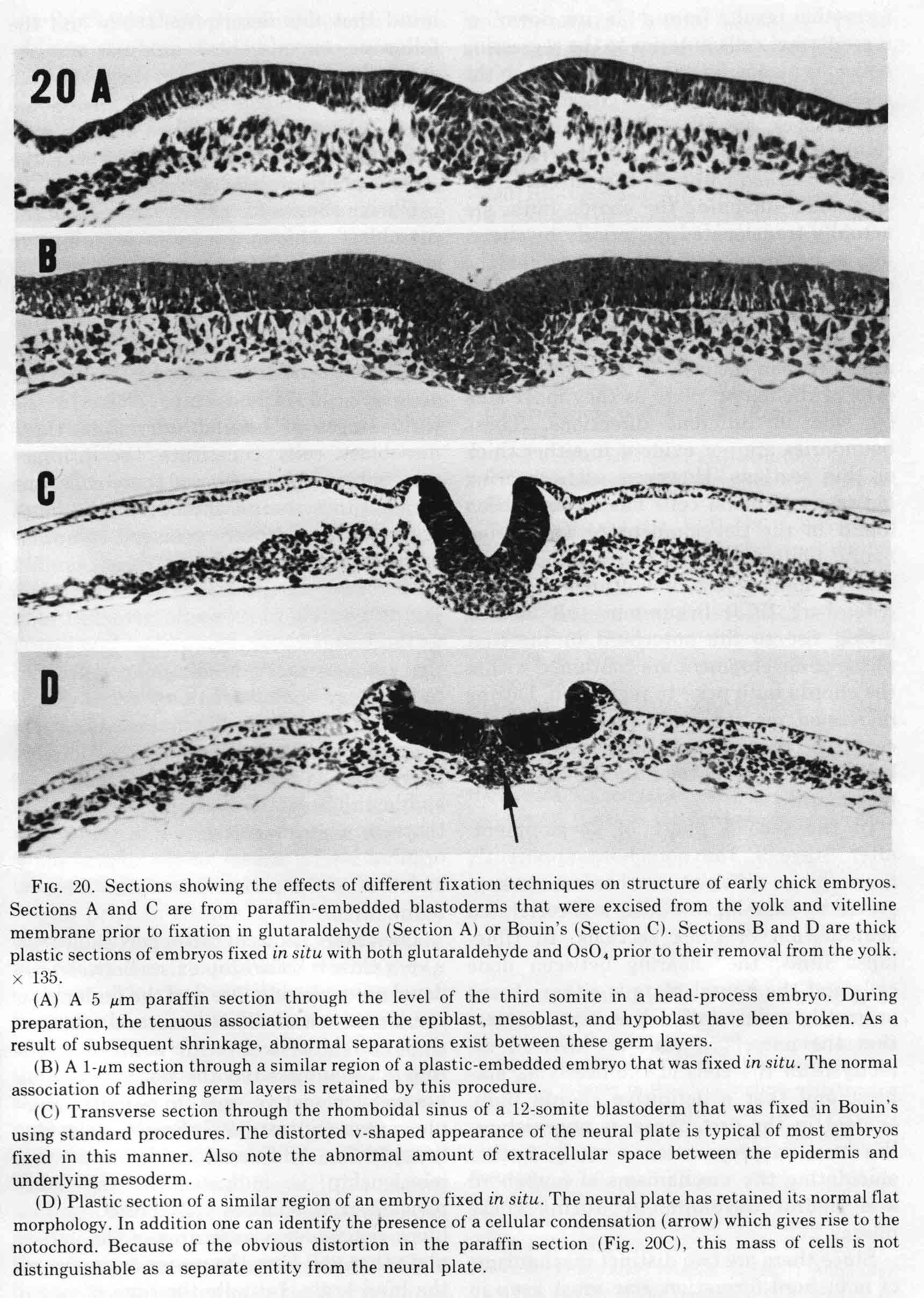
To illustrate the progression of formation of the presumptive somite, plastic thick sections of embryos fixed in situ on the yolk were used to avoid artifacts. Transverse plastic sections, shown below, were made through the level of the third somite in embryos ranging in age from primitive streak stage (stage 4) to 8-somite stage (stage 9). All sections are at the same magnification and their midlines are at the left. 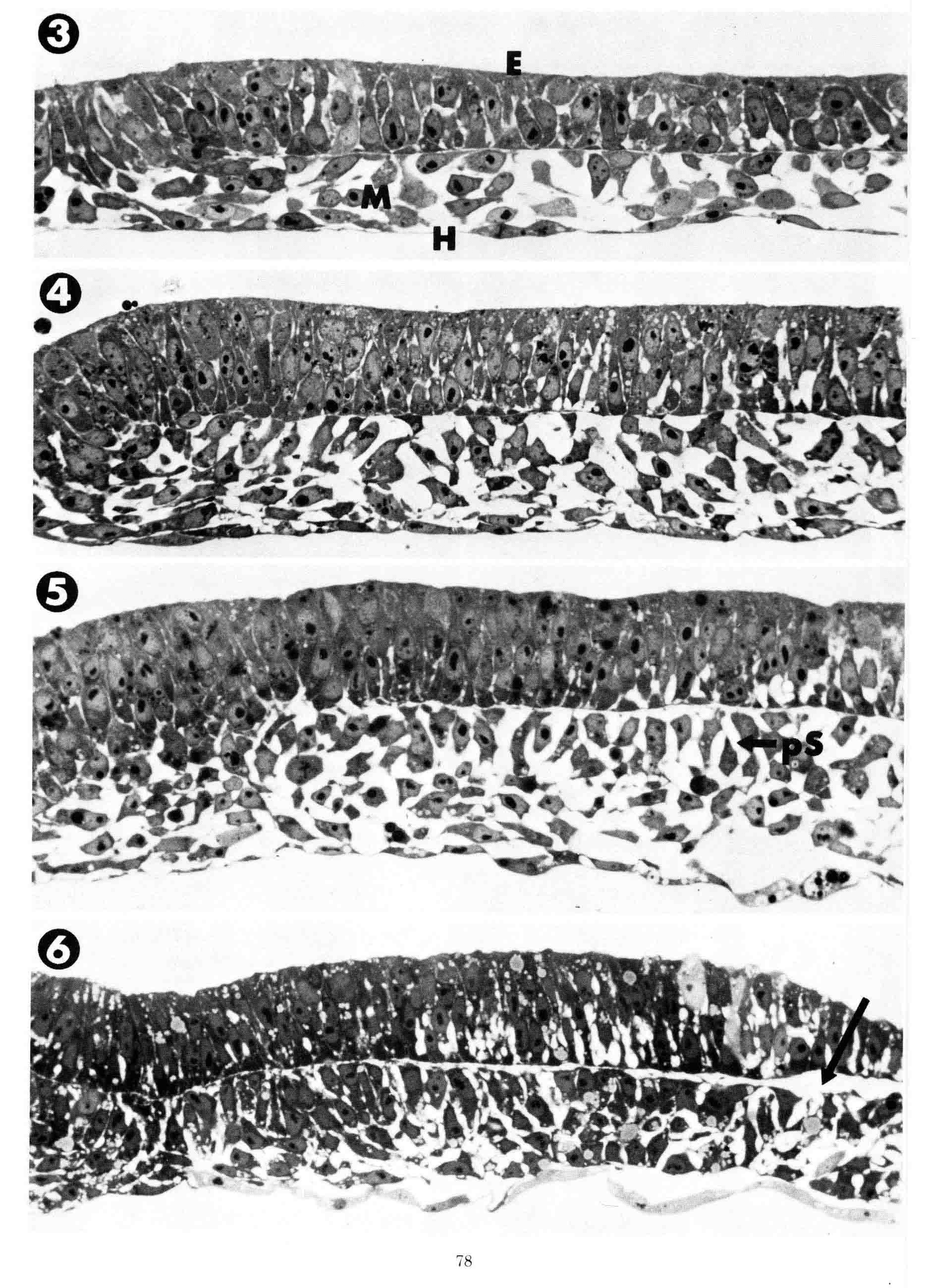
3 = Primitive streak stage. Mesoblast cells (M) were fixed during their lateral migration from the streak. The mesoblast cells attach to the overlying epiblast (E) or to underlying hypoblast (H) as they migrate. At this stage the epiblast consists of presumptive neural plate that is continuous with the primitive streak (left). The neural plate cells are organized as a simple pseudostratified epithelium. The neural plate has a flat basal surface and a basal lamina is present. The basal surface of the primitive streak is extremely irregular and uneven and from it cells appear to emerge and enter the mesoblast layer. 4 = head process stage. 5 = head-fold stage. During these two stages, both the overlying epiblast layer (future neural plate) and the mesoblast layer thicken as the neural plate condenses toward the midline. 6 = 1 somite stage. The neural plate has narrowed toward the midline so that the junction with the epidermis now appears in the section . The future somite mesoderm beneath extends laterally exactly to the border between the neural plate and the contiguous epidermis (arrow). Past this lateral point, the mesoblast no longer adheres to the overlying ectoderm. The sections above illustrate the process of appearance of the bilaminar somitomere which was defined years later. The sections below show the formation of the somite. 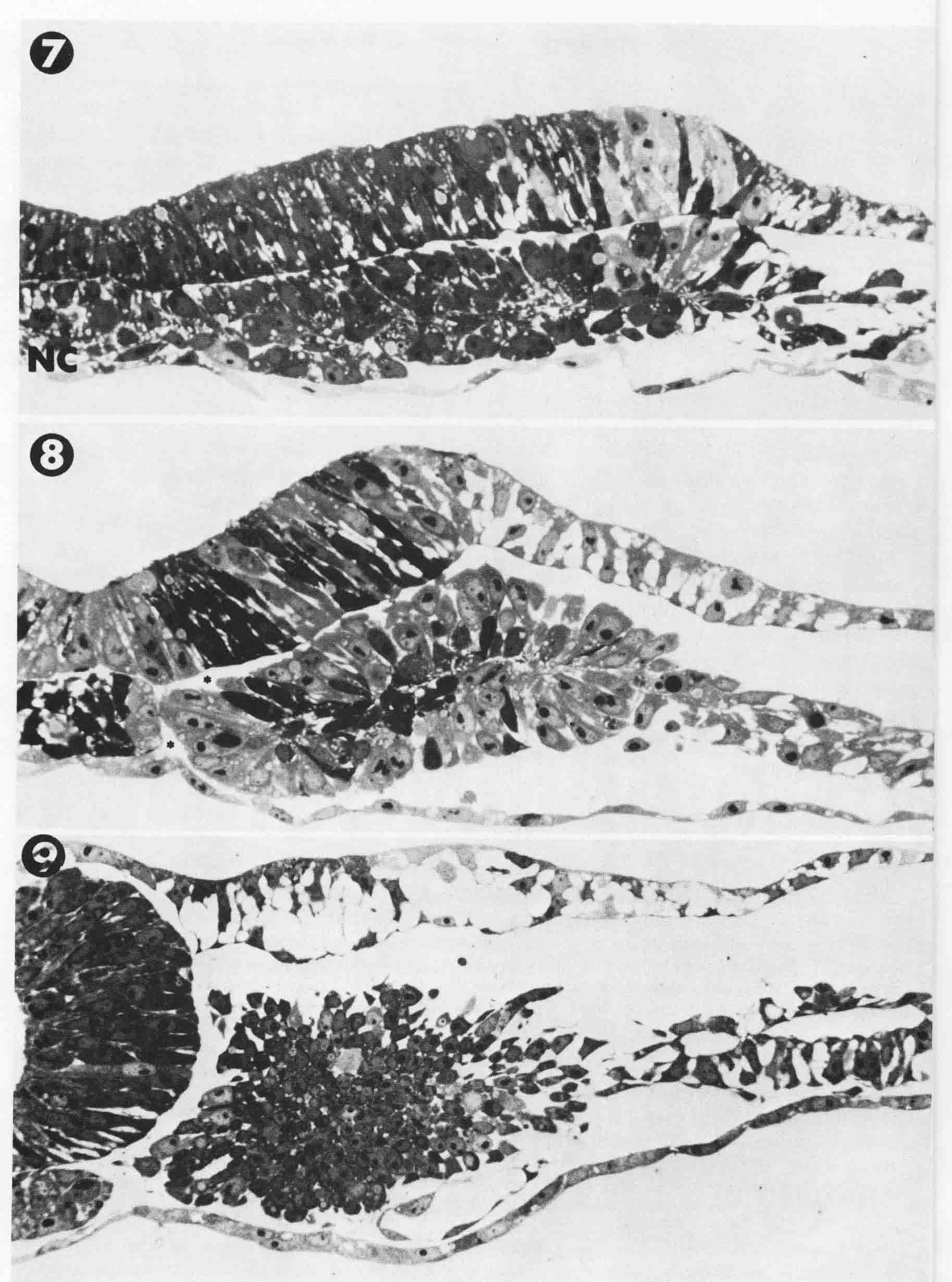
7 = 2 to 3 somite stage. At this stage, the anterior and posterior boundaries are just forming that define the third somite. At the midline (left), are cells of the forming notochord (NC). The neural plate has condensed more toward the midline, accompanied by a matching condensation in the underlying forming somite. 8 = 4-somite stage. The neural plate has lifted as tube formation has begun. The bilaminar somite beneath the neural plate has rolled into a rosette configuration. Adhesion between the neural plate and the somite persists, but space is found between the protruding portion of the somite and the overlying epidermis. 9 = 8-somite stage. The neural tube has completed closure. Electron micrograph figures (not shown) found fibrils bind the somite to the notochord across the space between them. Bruce Lipton's experimental analysis of somite formation gives evidence of several major conclusions about somite formation. First, there is a prepattern of segmentation in the mesoderm before regression of the node. Second, the regression of the node shears the mesoderm into right and left parts and releases the expression of the segmental pattern. Third, the notochord, which arises from node cells, is necessary to stabilize the forming somites by becoming attached to the somite mesoderm by collagen fibers. Fourth, the neural plate directs the mesoderm to form as presomite and somite. 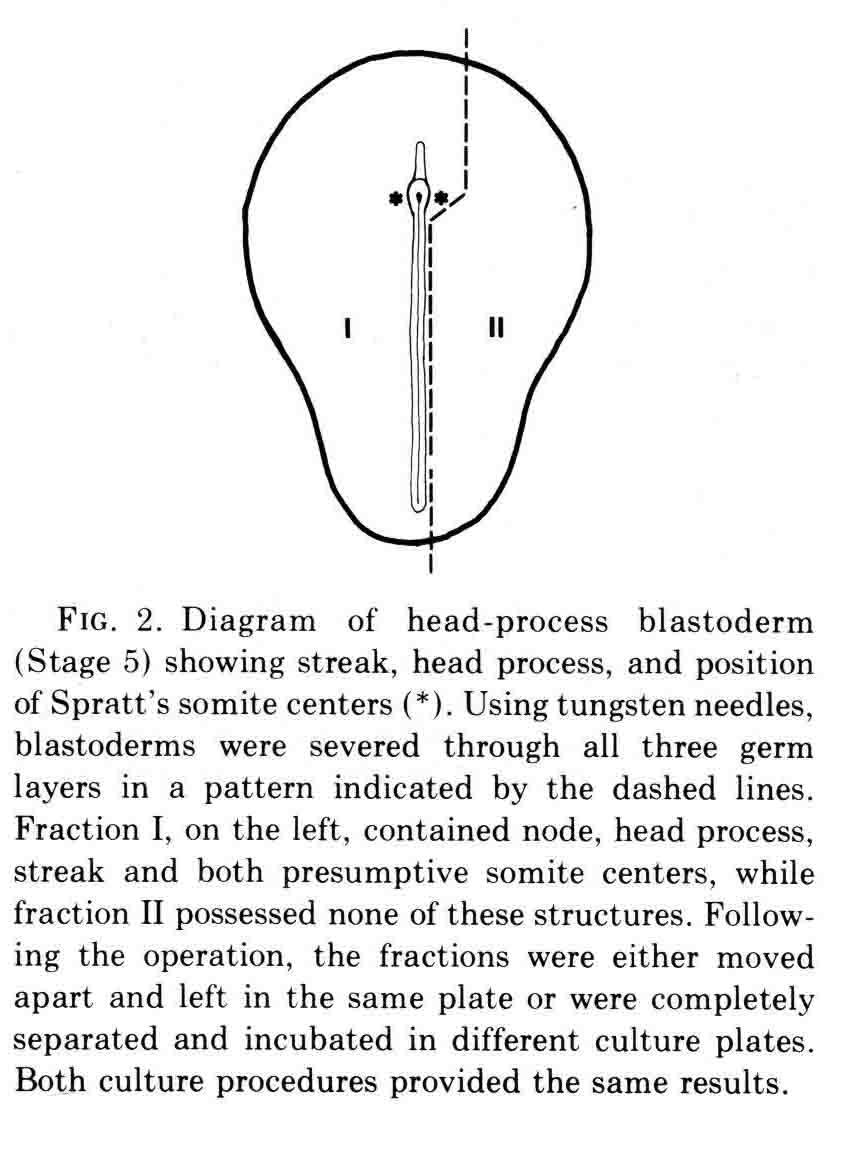
At the time this experiment was done, various authors had proposed that "somite forming centers", or primitive streak and regression, or node and notochord were necessary for somite formtion. All these parts are left in fraction I and fraction II contains none of them. We expected that only fraction I would form somites. However, both fractions made somites as shown in the figure below. 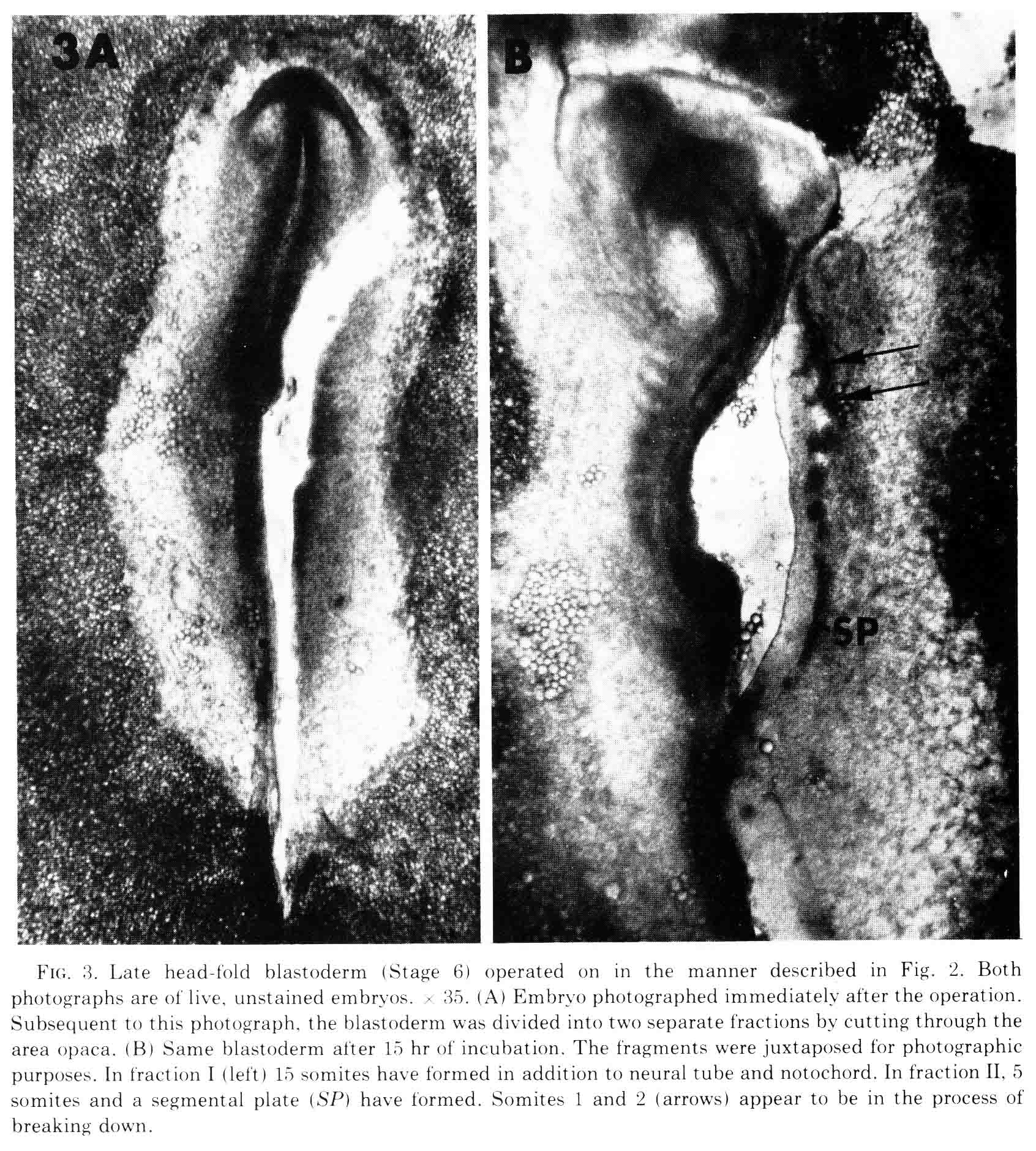
The figure shows the fractions just after they are cut, and after being cultured for 10 hours. The first two somites in fraction II appear to be in the process of breaking down. Fraction two has no notochord. When cultured for more than 14 to 30 hours, all the mesoderm migrates out of the explant, most likely because there is no notochord to which the somite can attach. This is shown in the figure below. 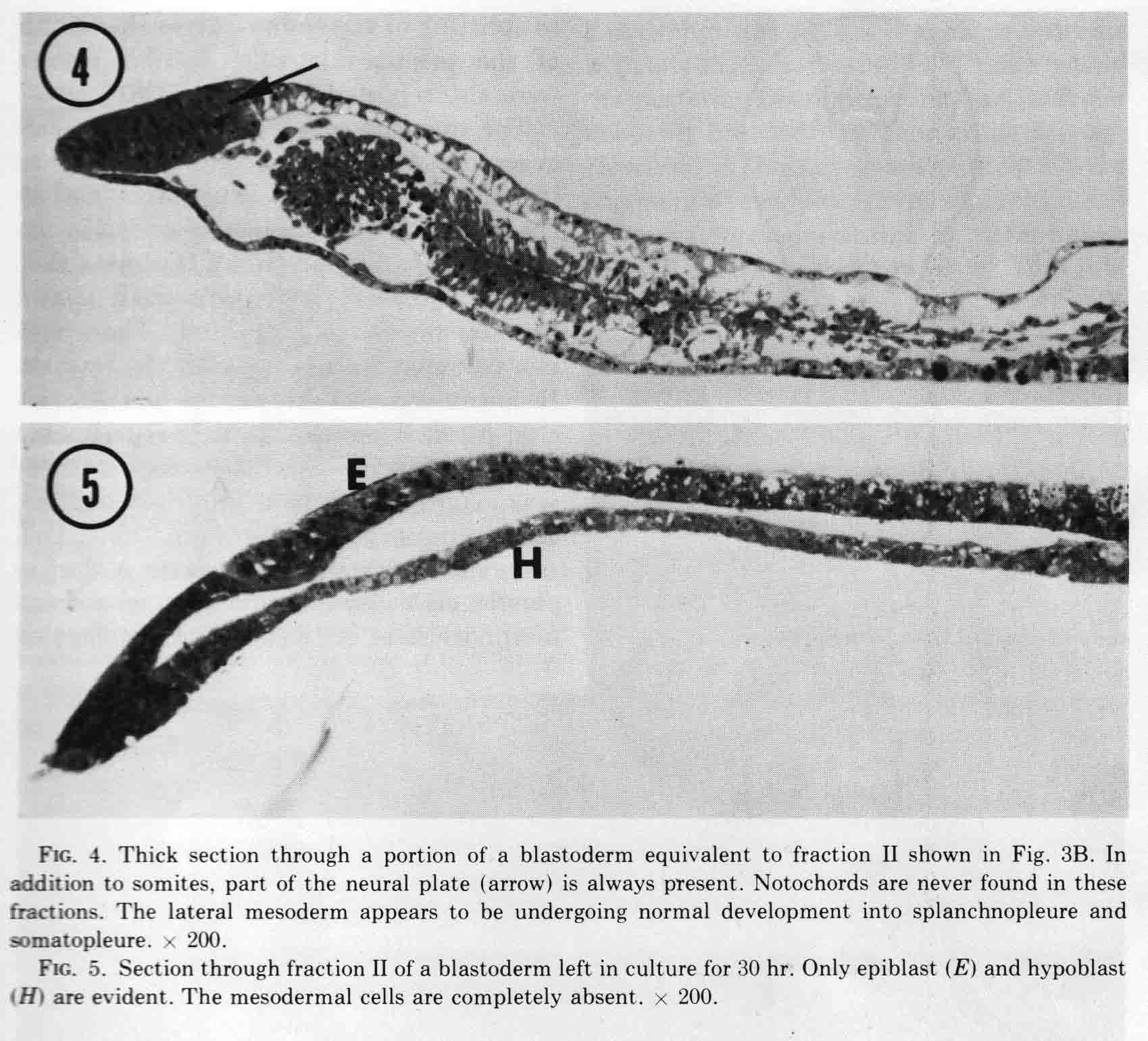
Finally, an experiment that directly shows the role of the neural plate in somite formation. 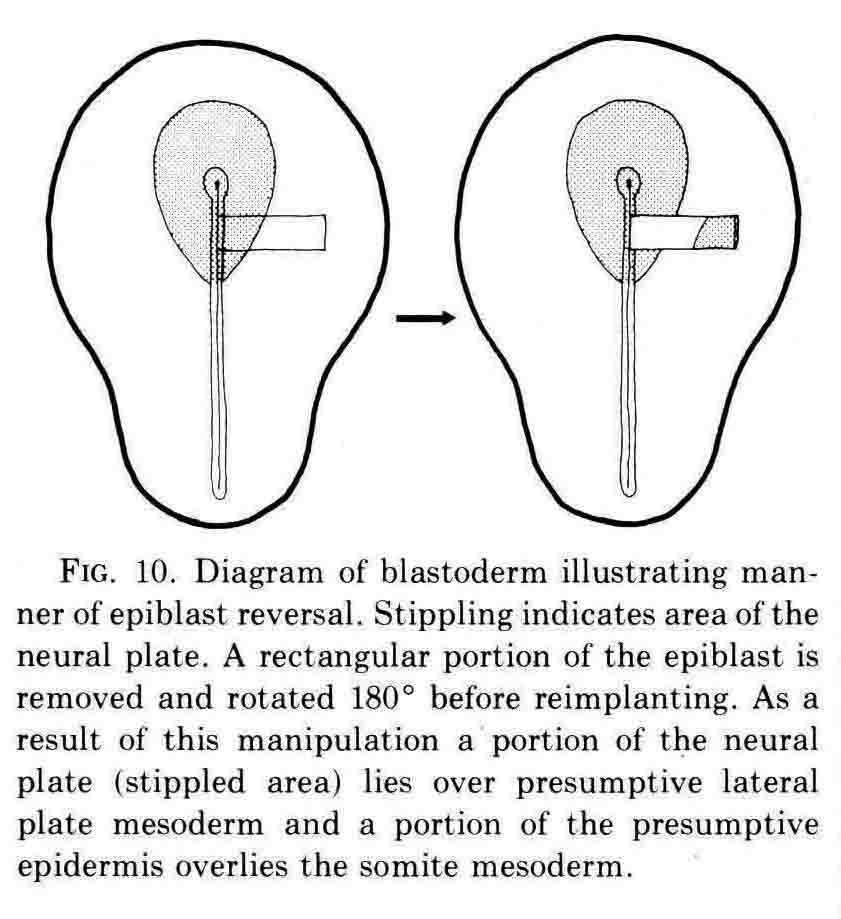
The rotation of the piece of neural plate and epidermis placed some neural plate over presumptive lateral plate mesoderm. After culture, somites formed to either side of the neural plate at left, and an extra somite formed below the displaced piece of neural plate. David Packard investigated how surrounding tissues affected somite formation from the chick segmental plate in embryos with from 8 to 20 pairs of somites (3). The segmental plate is the region between the last-formed somite and the regressing primitive node and streak. His work led to several important conclusions. First, the segmental plate of the chick embryo contains about 12 future somites. Second, the segmental plate can segment into its full complement of somites without further contact with the axial structures. Third, intimate continued contact between the segmental plate and axial structures, especially the notochord, is necessary for normal somite morphogenesis and stability. Some of these experiments are illustrated below. 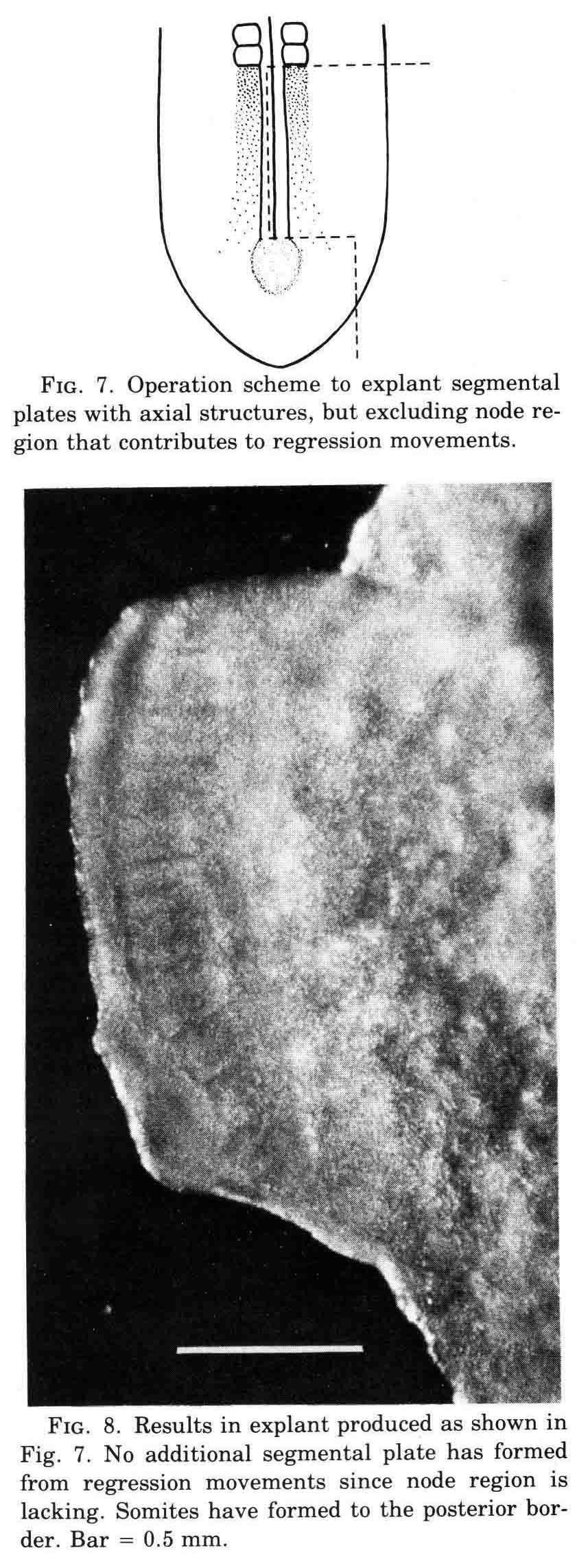
The experiment above illustrates that the normal complement of normal somites forms in an explanted segmental plate if the notochord is in the explant. 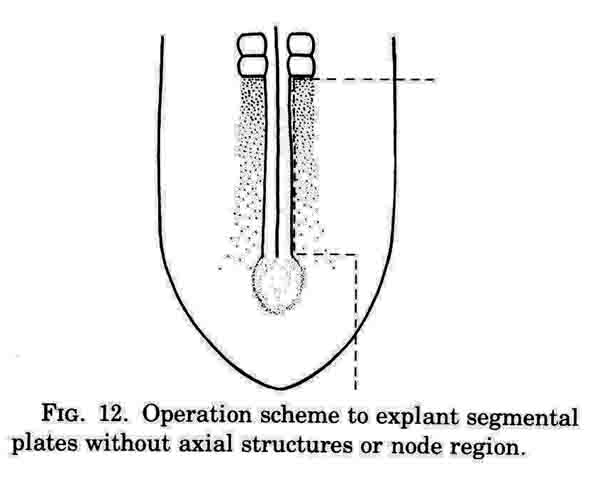
This operation excludes axial structures; containing just segmental plate, lateral mesoderm, along with neural plate and epidermis and hypoblast. 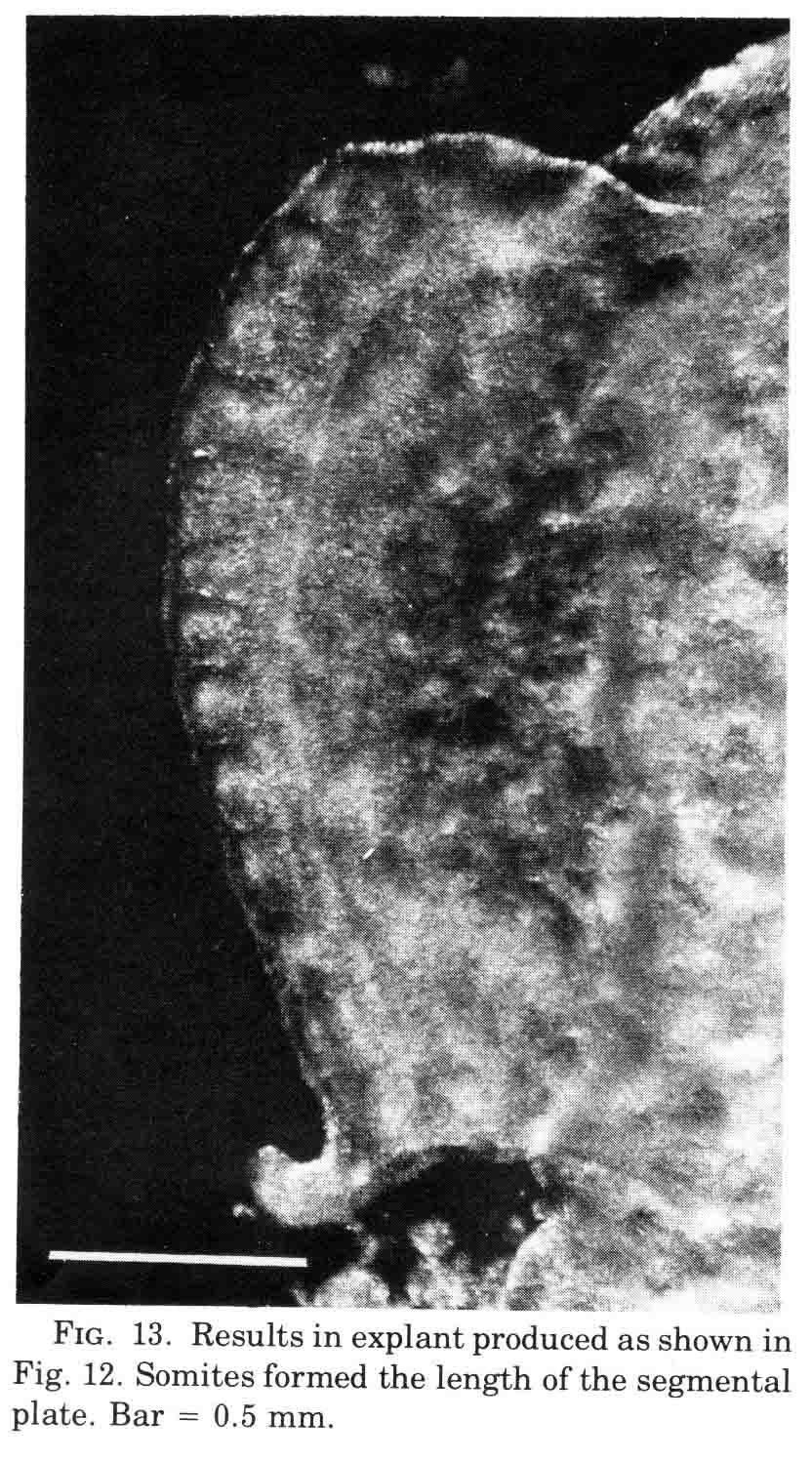
A normal complement of somites forms, but the morphological character of the formed somites is not normal. In the absence of axial structures, the somites that form assume a hollow morphology, and more posterior somites dissociate. The hollow morphology is illustrated in the section below. 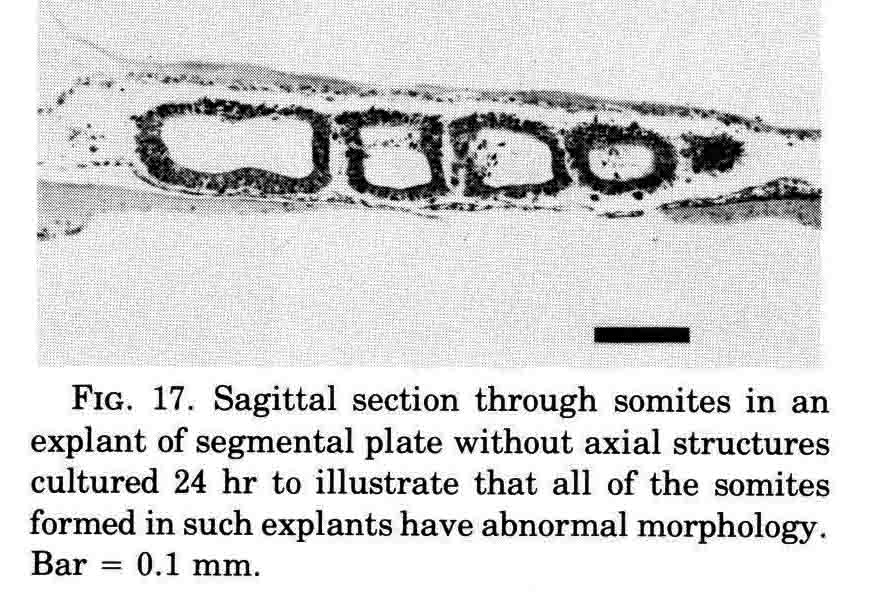
The work above was done before the discovery of somitomeres by Stephen Meier in 1979 (4). I have answered critics of somitomeres at the end of this discussion (17). A pdf of this paper is here . Stephen Meier accidentally discovered somitomeres when he stripped off the ectoderm of the head of a chick embryo and examined the head paraxial mesoderm with stereo scanning electron microscopy (SEM). Meier found metamerically arranged organised mesenchymal units he termed 'somitomeres' in both the head and the segmental plate of the chick embryo. The illustration below (from 4) gives a dorsal view and a ventral view of somitomeres near Hensen's node. The top stereo pair is a dorsal view of two somitomeres, each a circular swirl of mesenchymal cells. The bottom stereo pair shows a ventral view of a somitomere that has a central cluster of microvillous stubs. These clusters are also visible on the ventral surface of the adjacent hypoblast. 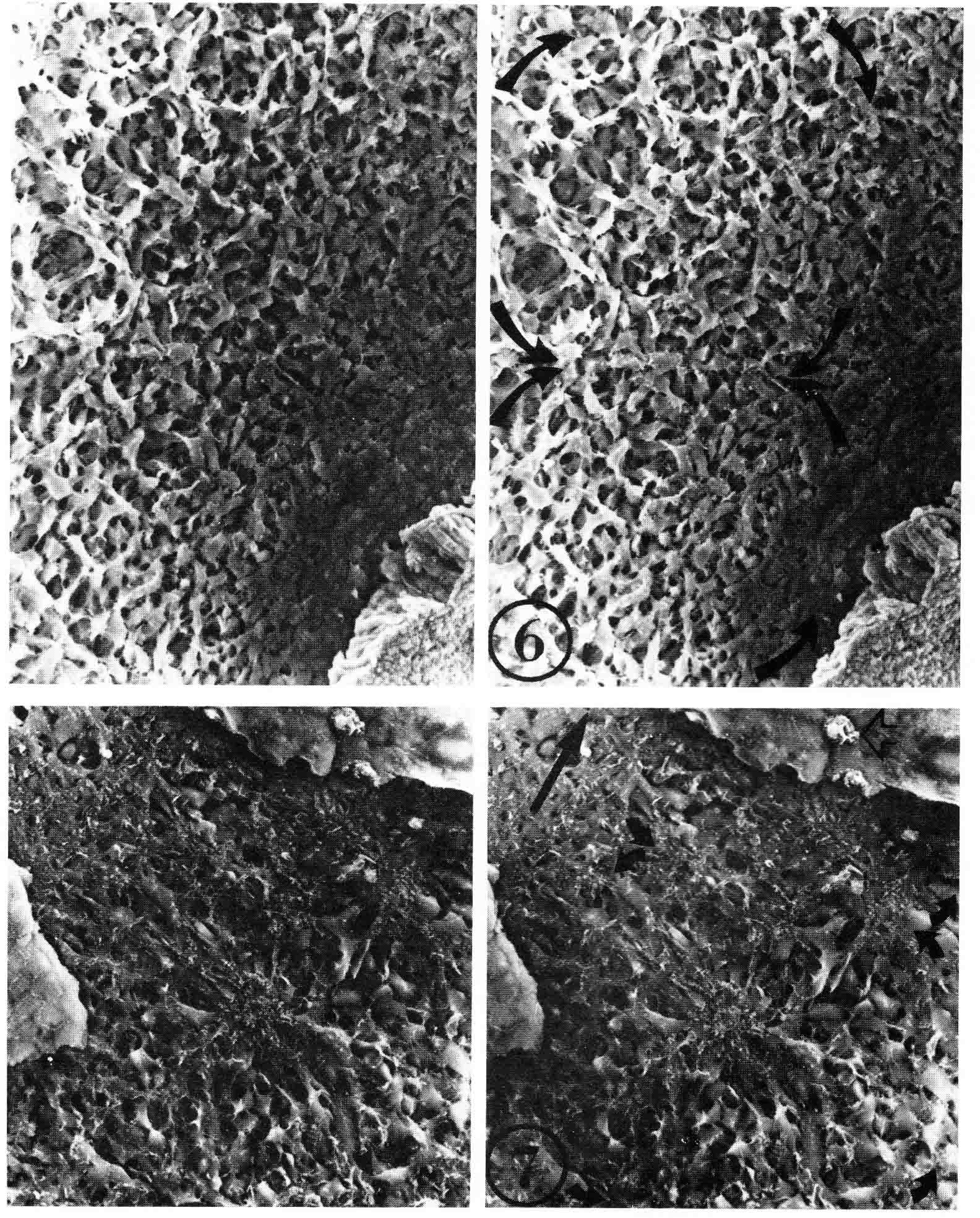
Somitomeres are found from the tip of the head to the tail. They form in pairs in strict cranial to caudal order. In the chick embryo, there are seven pairs of cranial somitomeres. Meier (5) illustrated the cranial somitomeres and the first somites (below). 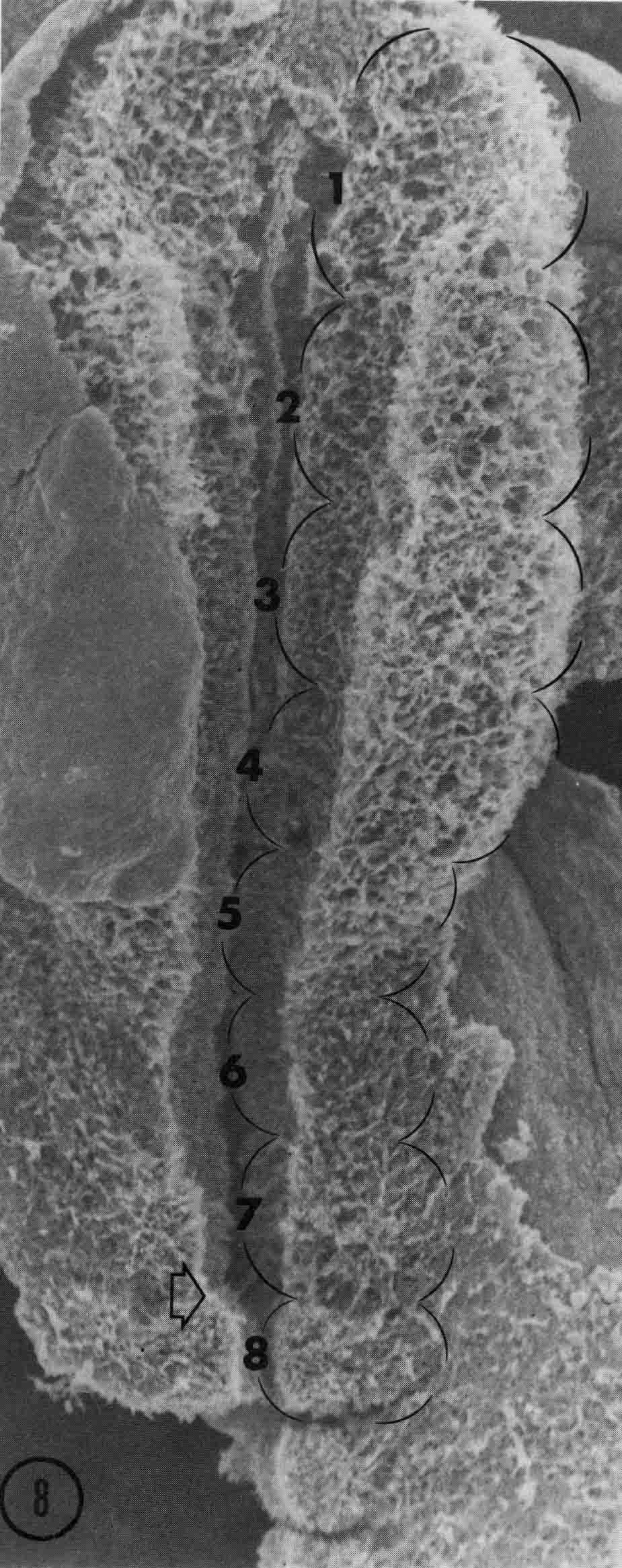
Segments 1 to 7 are cranial somitomeres; they do not condense into somites, but rather expand as the brain parts with which they are associated expand. Segment 8 is the first somite and appears just caudal to the otic (ear) ectoderm. Segments 8,9,10, and 11 are the occipital somites of the head. The segmental plate Is paraxial mesoderm lying between the most recently formed somite and the Hensen's node. Meier showed that the segmental plate consists of about 11 somitomeres. The early development of somitomeres in the chick embryo is shown in the diagram below from (6). 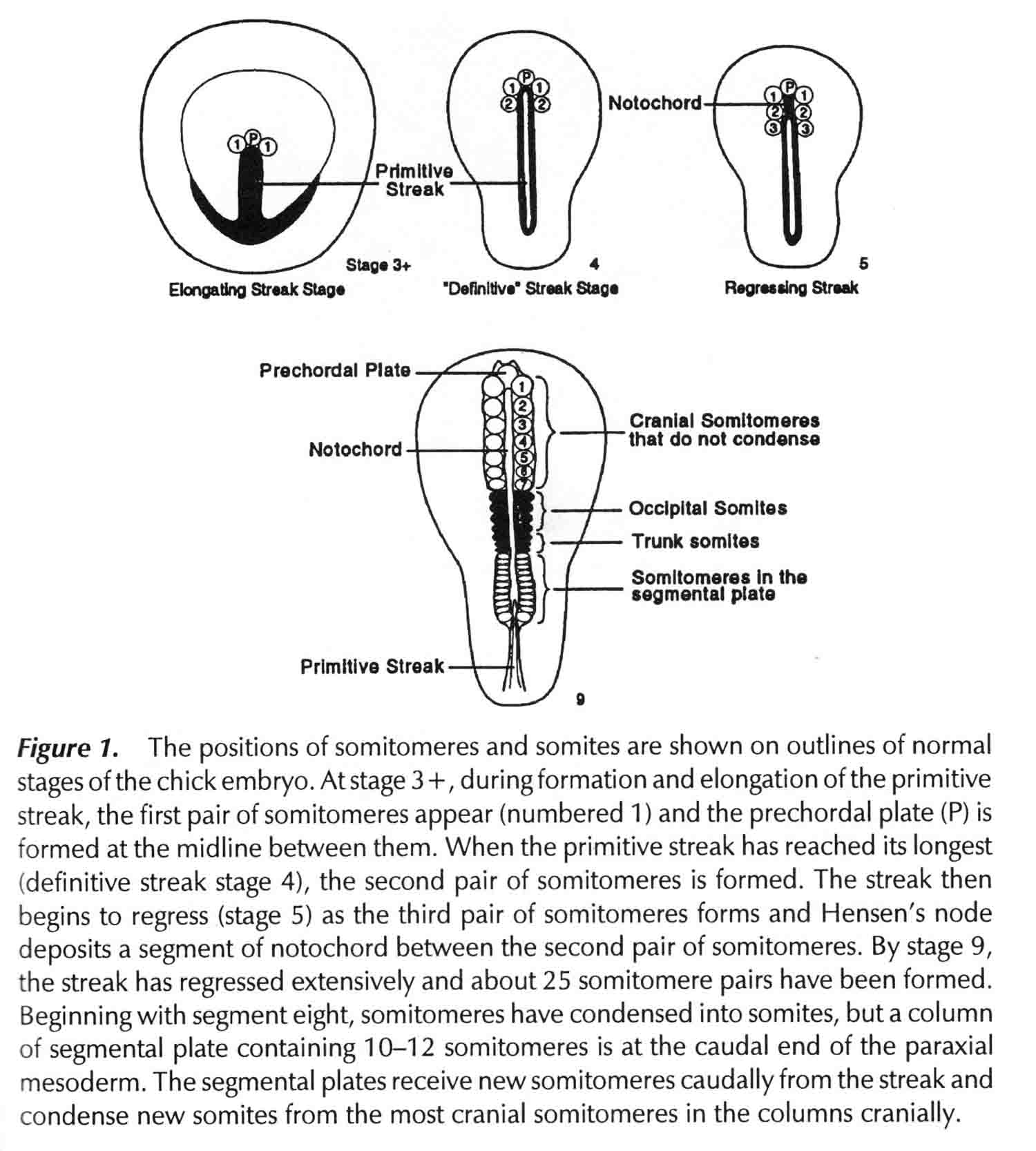
Packard and Meier (7) cut explants from chick and quail embryos as shown in the diagram below. 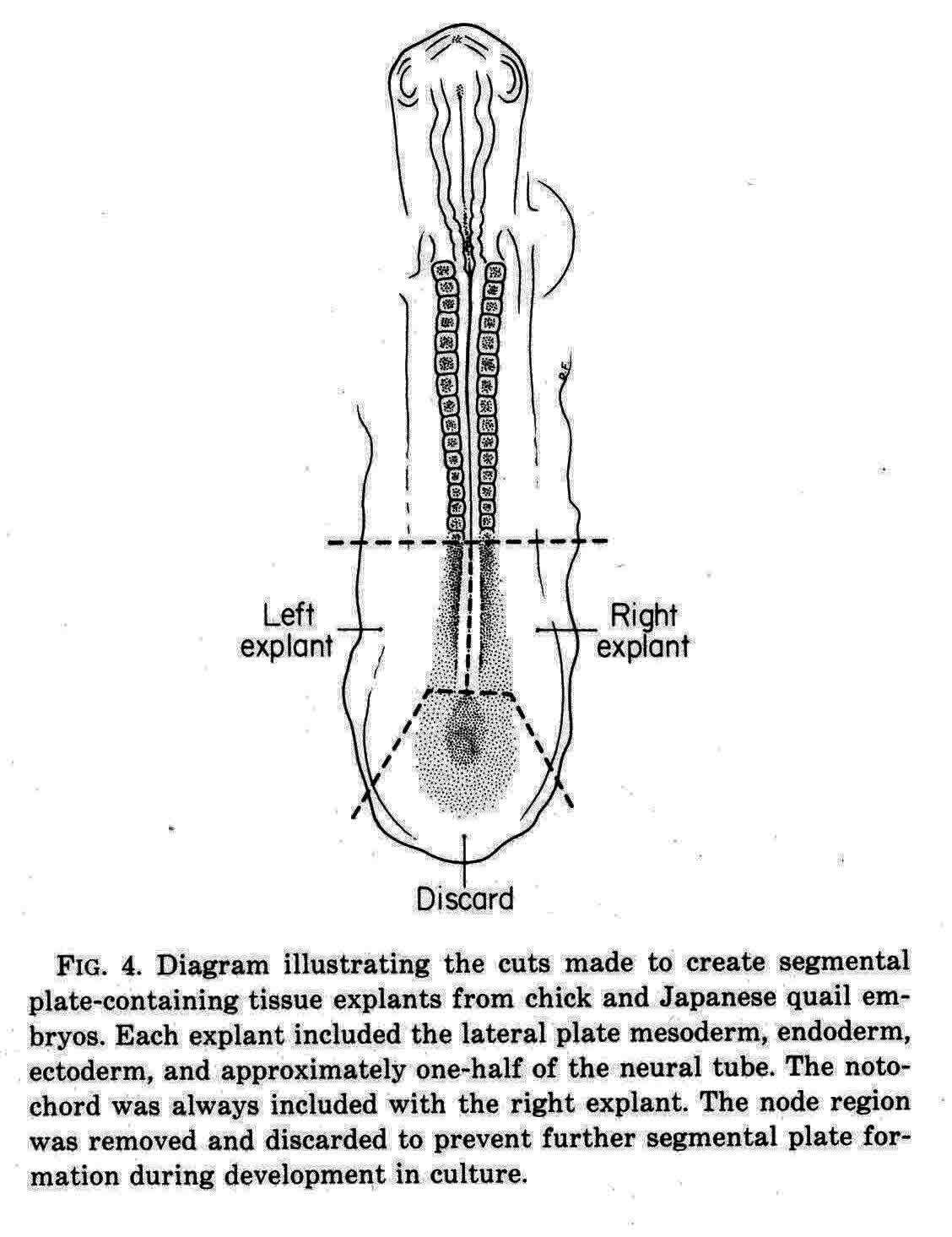
In one explant of each pair, they counted the number of somitomeres, which was found to be quite consistently 10.0 plus or minus 1.5, and the number was independent of the length of the segmental plate. The other explant of each pair was cultured for 10 hours when a number of somites had formed. The number of somites formed and the number of somitomeres left were then counted. The total was the same as the number of somitomeres counted in the other explant at the time of explant. For each somite formed in the explant, the number of somitomeres was reduced by one, thus somitomeres become somites. Somitomeres have been observed in several classes of vertebrates. They were discovered in the chick embryo (Gallus domesticus)(4), and then in another avian species, the Japanese quail, Coturnix coturnix japonica (7,8,9,10). Quail somitomeres are quite similar to those of the chick. Patrick Tam and Meier (11,12,13) examined somitomeres in a mammal, the common laboratory mouse, Mus musculus, CF-1 albino strain. Like the bird, the mouse has seven cranial somitomeres (numbers 1-7), and four occipital somites (numbers 8-11). In the mouse, the segmental plate is called presomitic mesoderm. Instead of having 10 to 12 somitomeres as in the bird segmental plate, the mouse has six somitomeres in the equivalent presomitic mesoderm. The illustration below (from 12 and 14) shows the cranial somitomeres of a mouse, and their relationship to the brain parts and neuromeres. 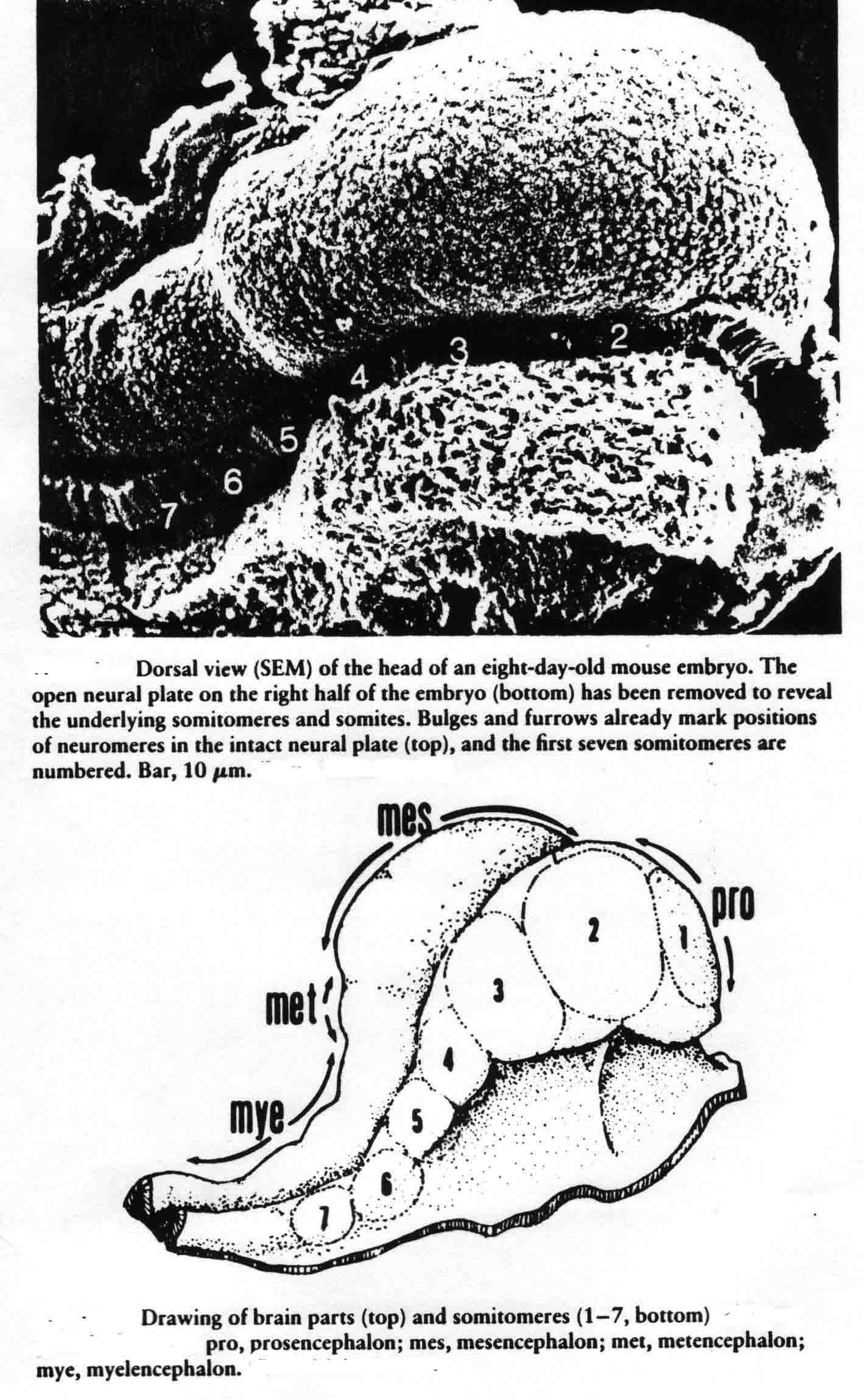
David Packard and Stephen Meier studied somitomeres in the snapping turtle (Chelydra serpentina )(8,9,15,16). The embryos of this turtle have the same number and arrangement of head somitomeres as in the bird and mouse embryos, but the segmental plate of the turtle has six to seven somitomeres as in the mouse. Before he died, Meier had made scanning electron micrographs of somitomeres of the whiptail lizard, Cnemidophorus uniparens, and I published an illustration of two somitomeres in the segmental plate from this lizard (17) shown below. 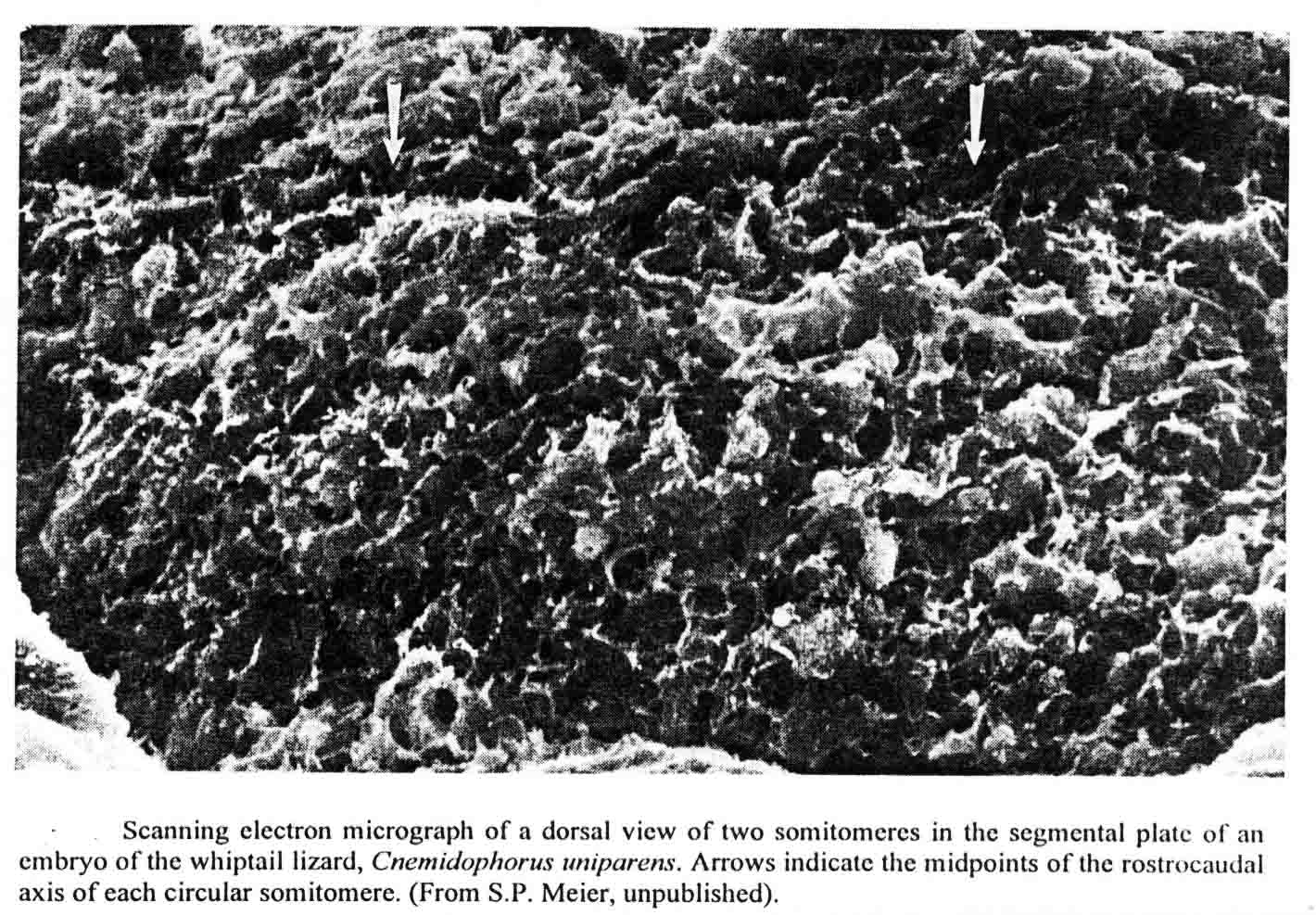
The birds, mouse, turtle and lizard discussed above are all amniotes, and all gastrulate using a primitive streak. Since somitmeres appear during gastrulation, Meier and I decided to look at somitomeres in amphibian embryos which gastrulate quite differently, using a blastopore. We have looked at a urodele, a west coast newt, Taricha torosa, and at an anuran, the common lab frog, Xenopus laevis. The cranial segments of the newt are readily apparent. The illustration below (from 18) is of a stage 23 (Normal Stages) embryo, which is the oldest stage at which the neural crest has not yet migrated over and obscured the somitomeres. The left side of the embryo is viewed in a scanning electron micrograph (X58) The epidermis has been removed. 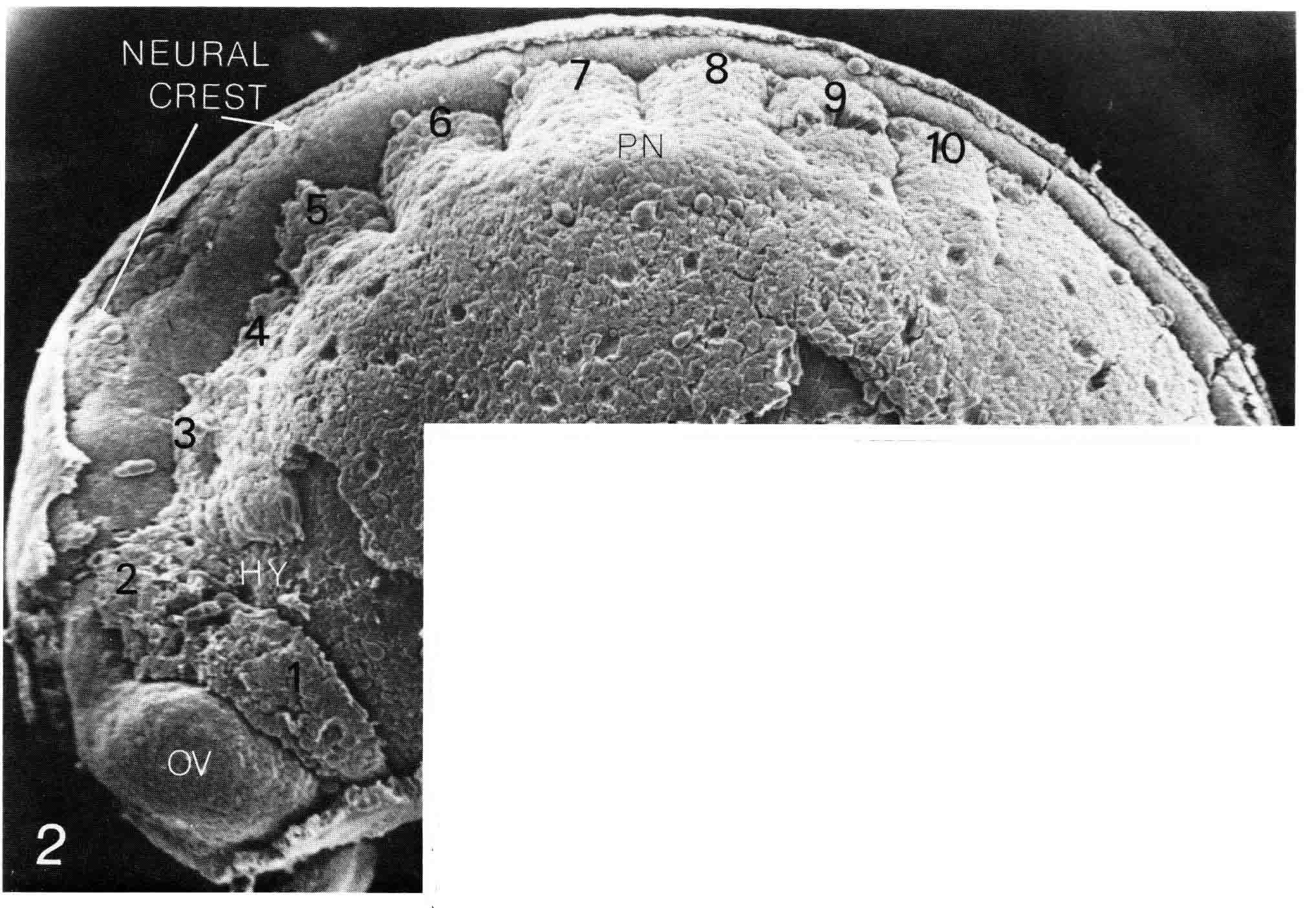
The newt embryo has but four cranial somitmeres (1,2,3,4). The otic vesicle (removed with the epidermis) lies above the fourth segment. The occipital somites consist of 5 and 6. Seven lies above the pronephric bulge (PN) and is thus the first spinal somite. Somitomere 1 is adjacent to the entire forebrain and its optic vesicle (OV). Somitomere 2 is adjacent to the entire midbrain. Segments 3,4,5,and 6 lie adjacent to the hindbrain. By this stage, the embryo has formed four trunk somites (7.8.9.and 10), followed caudally by the segmental plate. The number and arrangement of the cranial somitomeres in the newt are very similar to those of a shark as shown by Goodrich (19) below. In ech case, the otic (ear) vesicle lies above segment four. 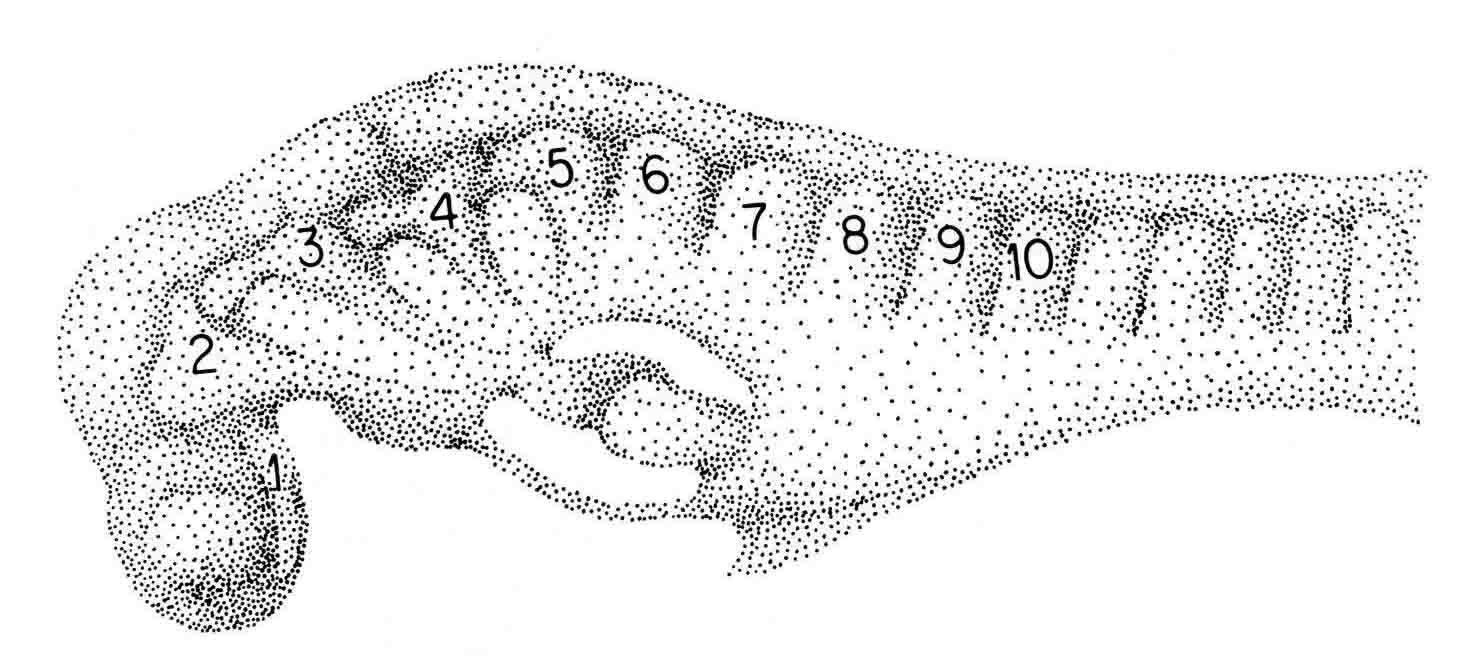
The number and arrangement of cranial somitomeres in the frog Xenopus laevis is the same as in the newt and shark. The otic vesicle is associated with segment 4, and segments 5 and 6 are occipital somites. A dorsal SEM view of a Xenopus embryo, stripped of its epidermis and neural plate is shown below (from 20). 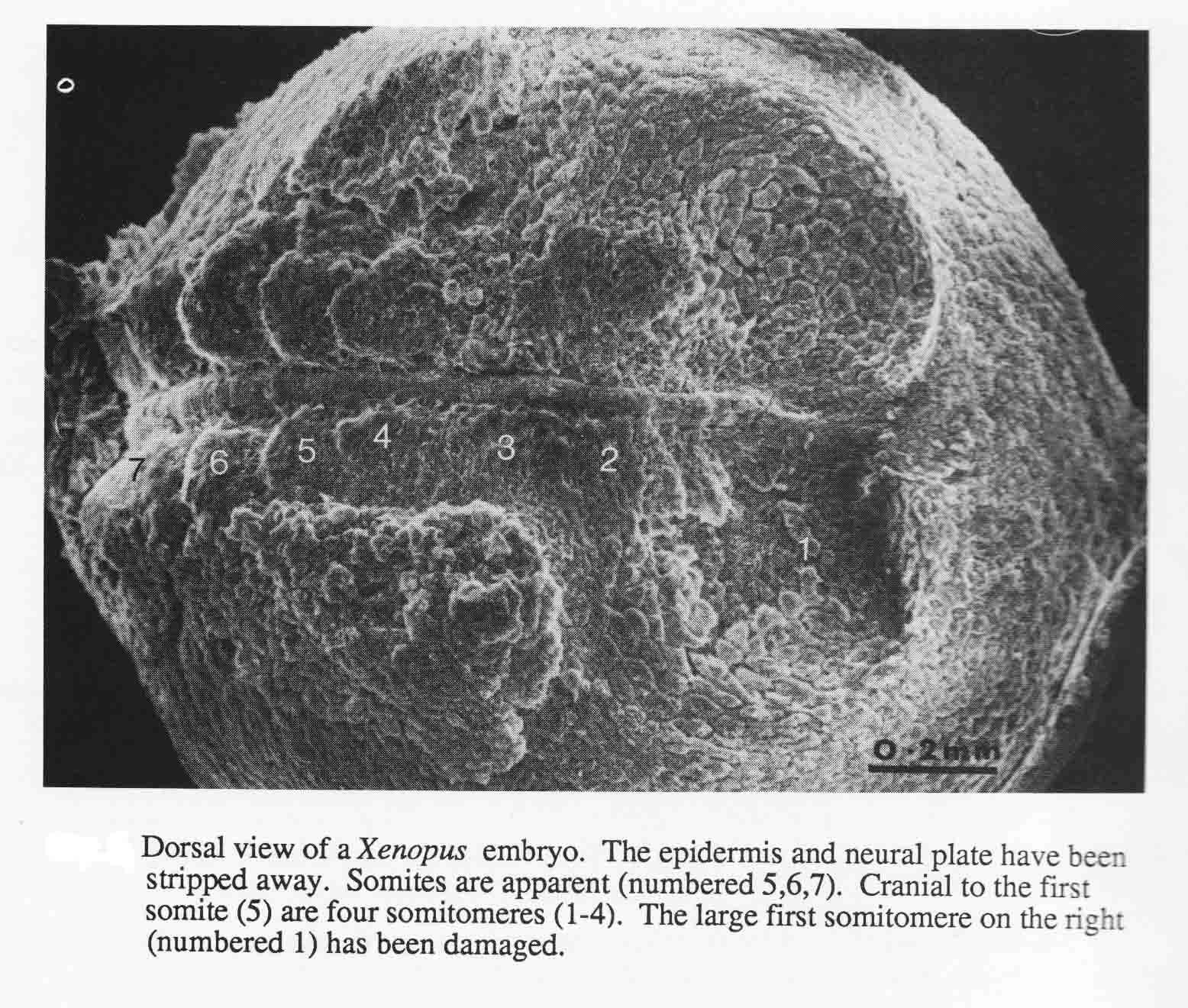
Embryos of the convenient laboratory cyprinodont, Oryzias latipes, the Japanese medaka, were studied to examine the somitmeres of a teleost (20,21). The diagram below summarizes the teleost somitomeres (from 21). 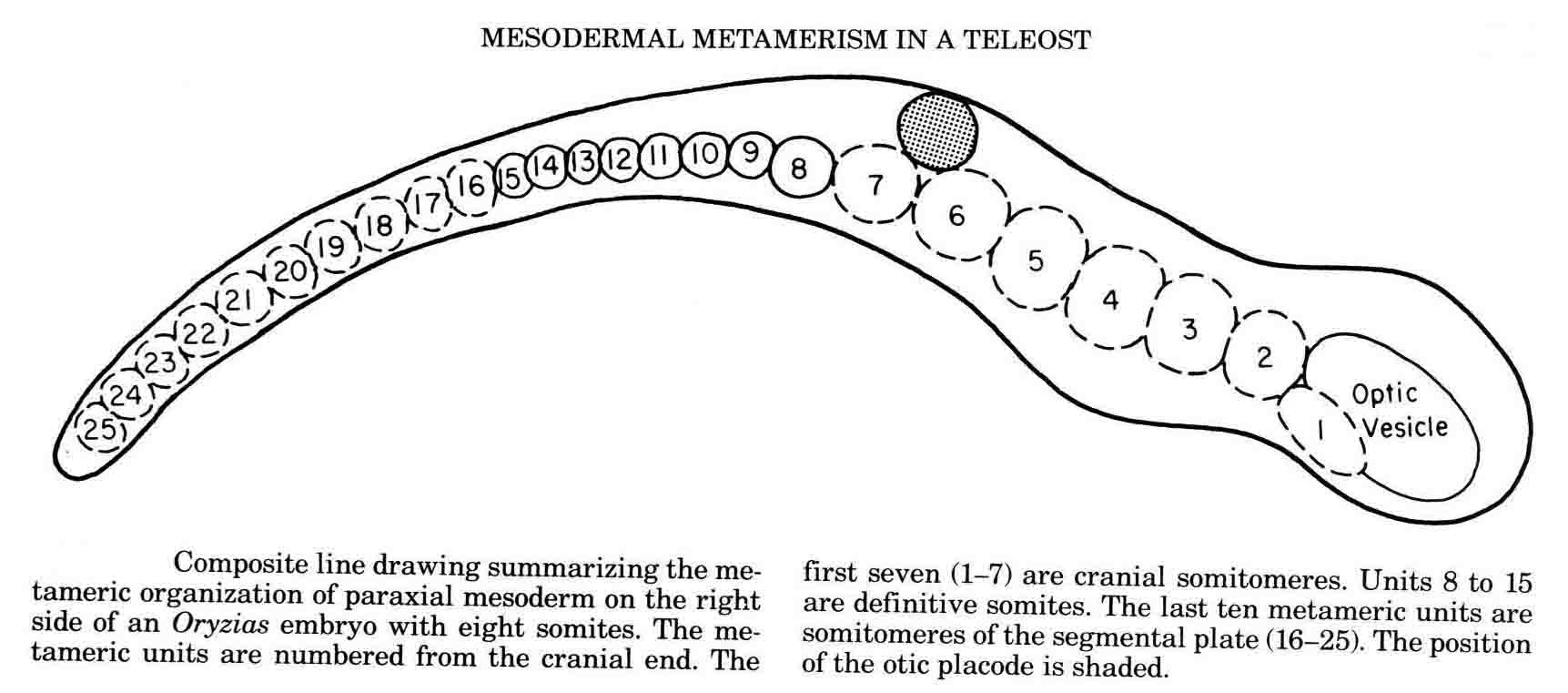
The illustration below shows somitomeres in the segmental plate of the medaka (top), and in the cranial area (bottom). The arrows from the diagram indicate the areas shown. 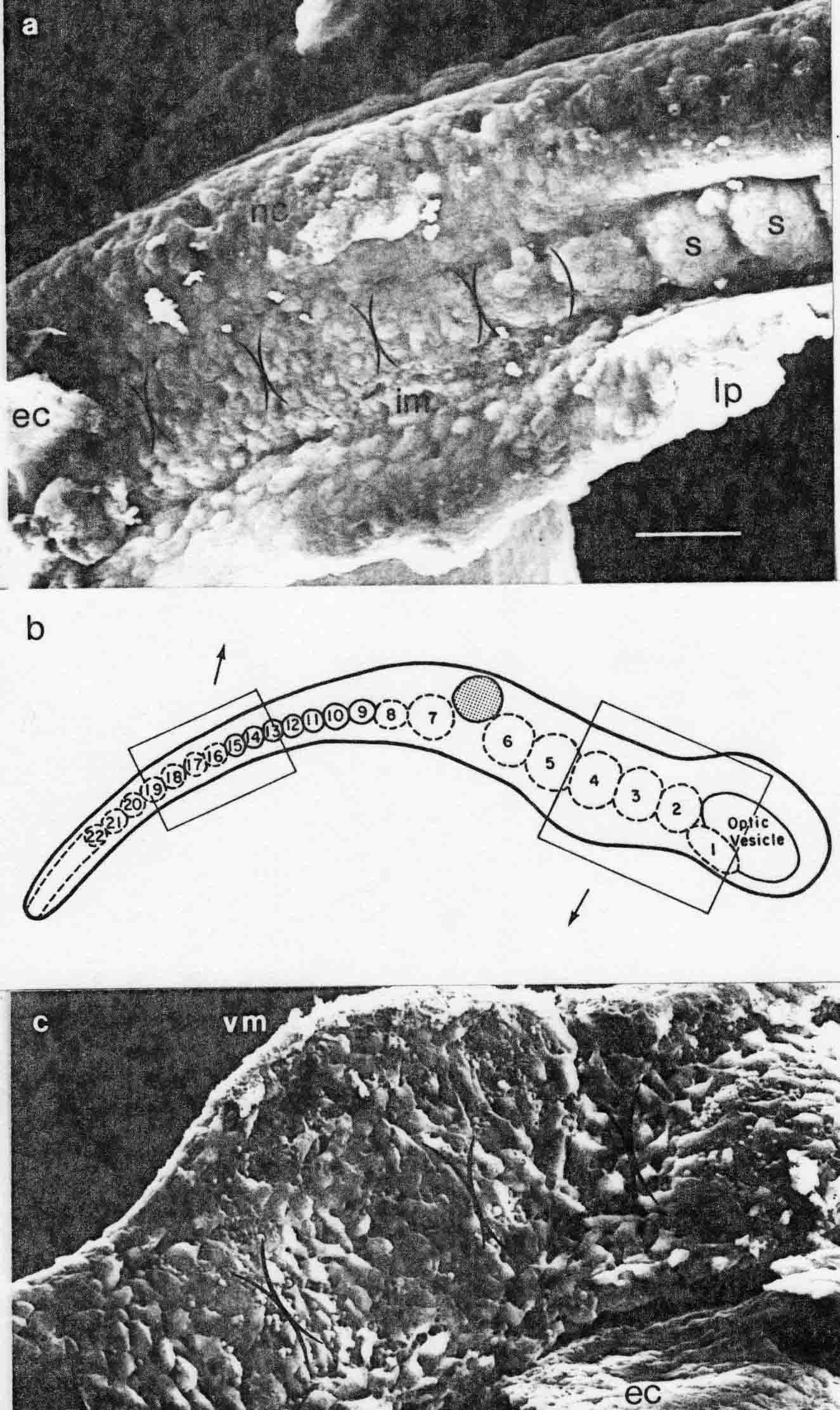
This teleost gastrulates differently from either the amniotes or the amphibia. The pattern of somitomeres in this fish is most like the pattern in amniote embryos. There are 7 cranial somitomeres, with the otic vesicle lying above segments 6 and 7, and 4 occipital somites. The segmental plate contains 10 somitomeres. The patterns of somitomeres among the classes examined are compared in the diagram below (from 14). 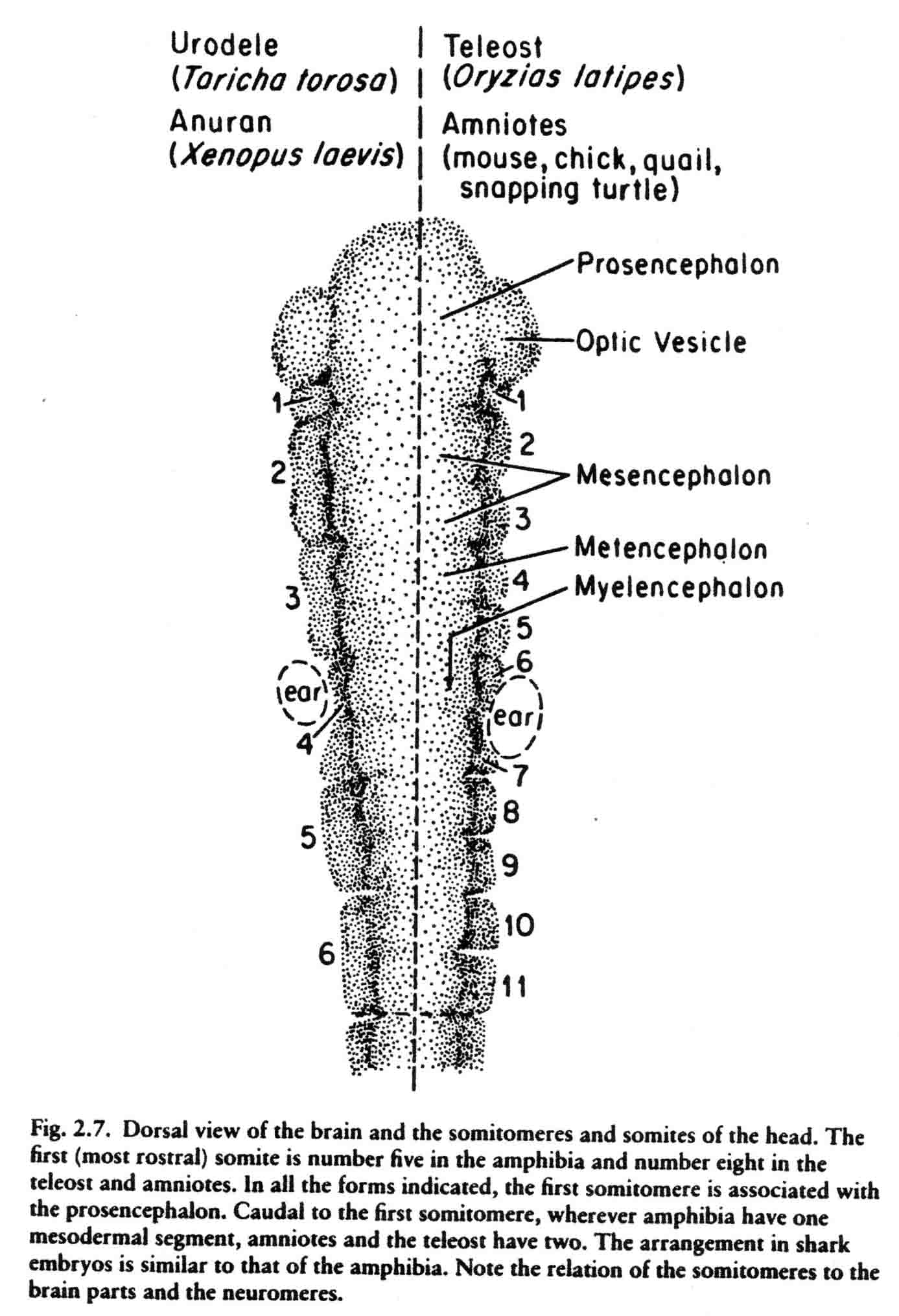
The first cranial somitomere is similar in all the taxa. The first somitomere abuts the entire forebrain (prosencephalon). The second somitomere of amphibia (and shark) abuts the entire midbrain (mesencephalon). In amniotes and a teleost, two somitomeres (2and 3) abut the midbrain, one next to each of the two mesomeres. From somitomere two on, where the amphibia have one somitomere, the amniotes and teleost have two. These studies lead to the question of what is the basal number of sub- and preotic somitomeres, four or seven? A study of the head segments of the embryo of the lamprey has been made by Damas (1944). The diagram below is taken from Damas (29) and appears in my most recent review (Jacobson, 2001) (17). Most likely the primive number of sub- and preotic somitomeres is four. 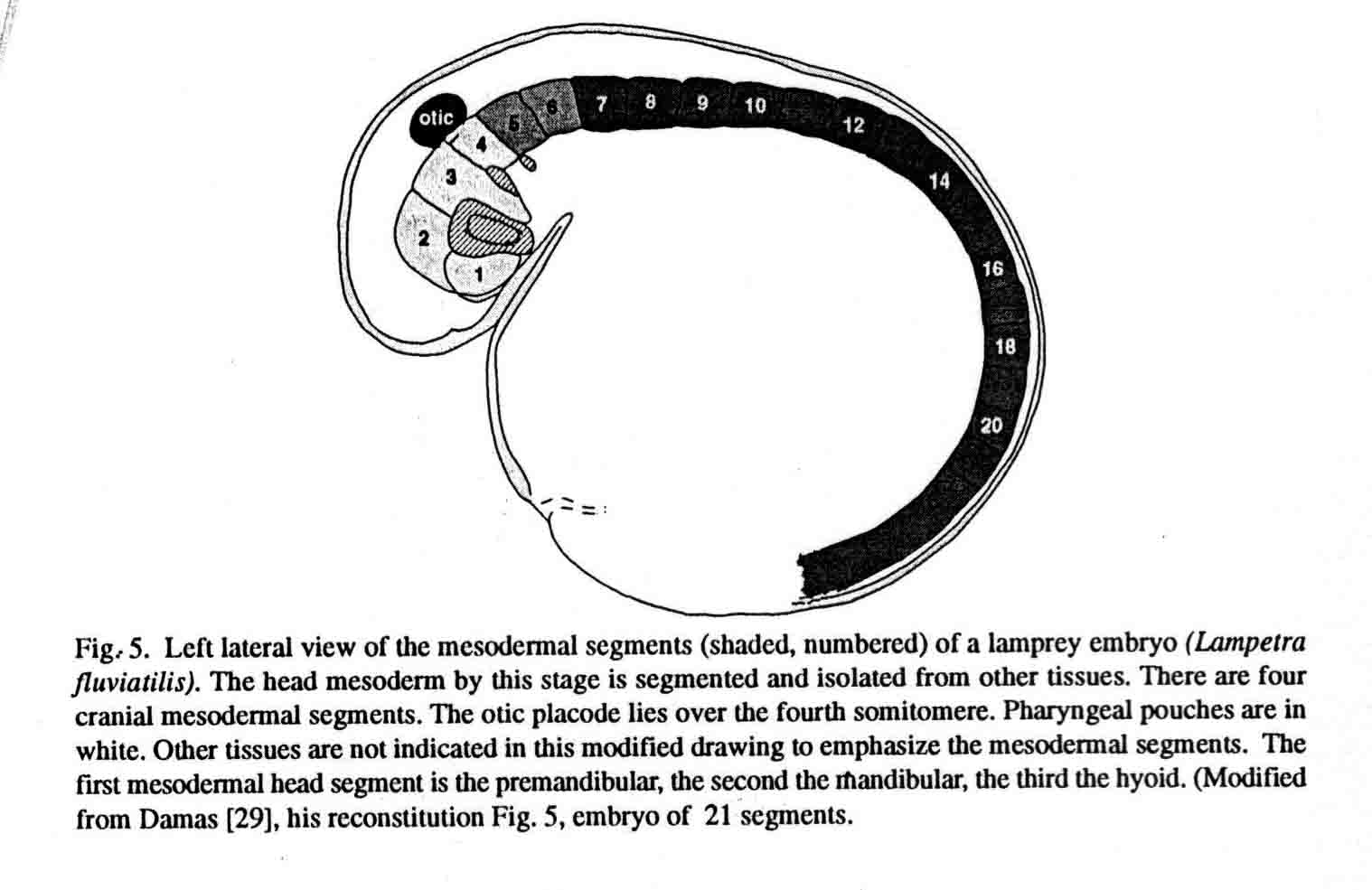
All somitomeres begin as expansion figures, and in the case of the cranial somitomeres, they continue to expand as the brain expands. Occipital somitomeres condense into somites, but are not attached to the notochord as well as are more posterior somites, and the more cranial occipital somites often disperse after forming. Lipton and Jacobson (2) pointed out that somites that form, but that do not bind to the notochordal sheath will disperse. They noted that in the chick embryo, "During development, the rate of somite formation lags behind the posterior regression of the node, resulting in a considerable distance between the last-formed somite and Hensen's node. This lag could allow the notochord to differentiate before somites form adjacent to it. This developmental sequence would ensure the stability of newly formed somites. The result of precocious segmentation may be reflected in the normal fates of somites 1-3. These somites form immediately after being bypassed by the node and prior to differentiation of the notochord. It is perhaps due to the absence of some stabilizing factor provided by the differentiated notochord that a substantial number of somitic cells disperse laterad to become part of the head mesenchyme. The loss of cells from two or three anterior somites is, in fact, so great that these are referred to as rudimentary (22)." Somitomeres are circular entities within rectangular mesodermal regions. There are crotches of cells between the somitomeres. These are illustrated below (17). 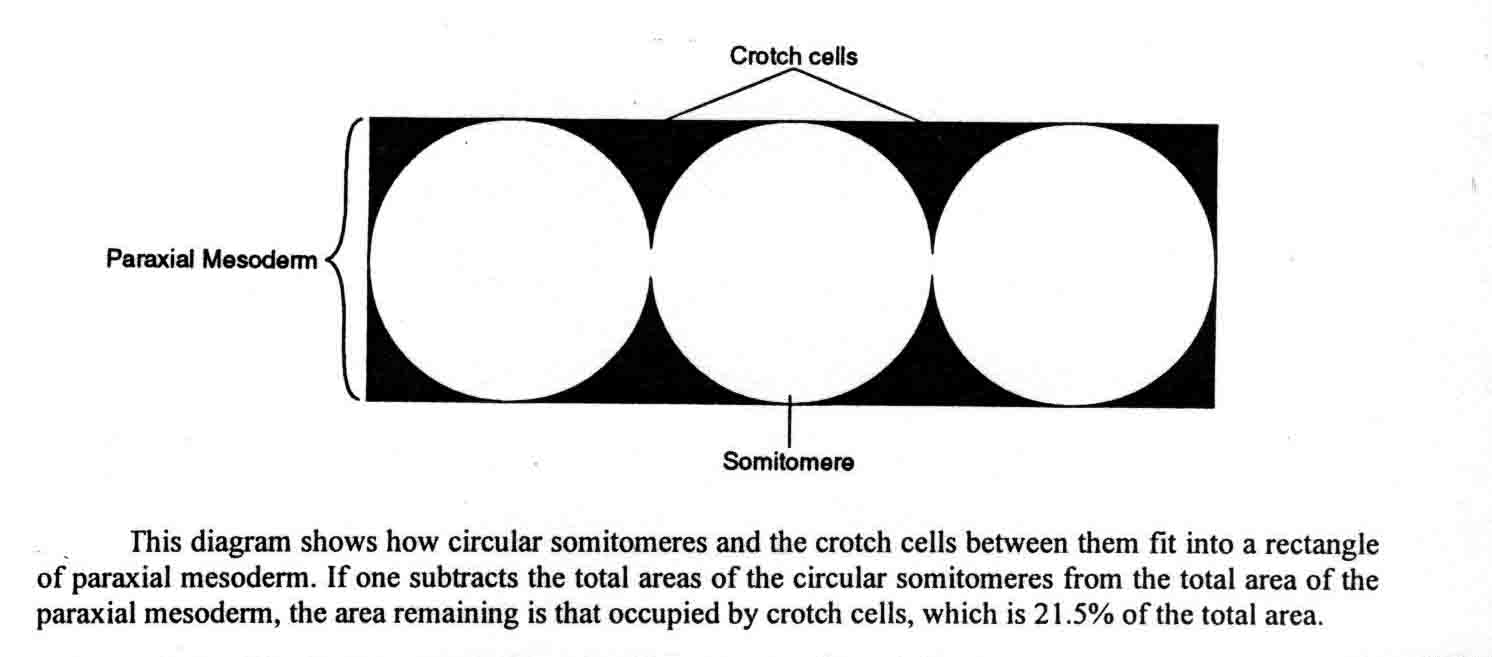
What becomes of these crotch cells in the paraxial mesoderm? Jacobson and Meier (18) proposed that these cells are mostly prospective vascular endothelial cells (angioblasts). These crotches of cells are found between the cranial somitomeres as well as in the segmental plate. Marjorie England (26) found intersegmental arteries between somitomeres within the entire segmental plate of chick embryos. She identified the arteries both with SEM and by injecting ink into the dorsal aorta. These intersegmental vessels are in the ventral crotches between somitomeres. This helps make clear that somitomere boundaries are real, and that their crotches likely contribute angioblasts. Cleaver and Krieg (27) identified angioblasts with flk-1 expression at the ventral edge of somites of Xenopus. The angioblasts migrated to the midline along a concentration gradient of the diffusible form of vascular endothelial growth factor which is emitted by the endodermal hypochord. The hypochord lies beneath the notochord. At the midline, the angioblasts form the dorsal aorta. Many studies indicate that vascular endothelial cells can arise from somites, for example, Cox and Poole (28). Noden (30) discusses the origen of angioblasts from the paraxial mesoderm of the avian head. By transplanting somitomeres between quail and chick embryos and using the quail nucleolar marker to indentify the cells from the quail transplants, Noden (23,24) found that the voluntary muscles of the head come from somitomeres. Somitomeres also contribute to cranial skeleton, and to dermis and meninges. Somitomeres thus make similar contributions in the head that somites make in the trunk. (Couly et al. (25) contend that somitomeres do not contribute to dermis.) In this final section on somitomeres, I give my answers to those who dismiss somitomeres. These answers were published in my latest review of the subject (17), and I quote from that source: "Some published accounts question the existence of, or the relevance of, somitomeres. Stern and Keynes (31) make the assumption that a metameric pattern of somitomeres requires that there be little or no cell movement within the segmental plate. or at least any movement be restricted to within somitomeres. They say, based upon unpublished observations, that this is not the case. Being mesenchymal cells, the cells of somitomeres naturally move about. This is a general characteristic of vertebrate embryonic tissues, including even cells in epithelial tissues such as the neural plate and tube in which changing of neighbors of cells is extensive (32). Mesenchymal cells of the limb bud move around. but no one denies their leg. The presomitic mesoderm is a morphologically patterned field of somitomeres which is mostly presumptive somites. It should be expected that regulation would occur in such a patterned field. When chick segmental plate is cut into bits, and fragments are mixed (33,34), the pattern of somites that emerges is normal. Also, extirpation of future intersomitic furrows from chick segmental plates did not prevent normal somite pattern (35). Many patterned mesenchymal groupings emerge within fields of other mesenchymal cells. Such patterns likely arise as groups of cells sort out from the surrounding cells be acquiring differences in cell surface adhesion molecules." Do somitomeres exchange cells? "One study (36) labelled paraxial mesenchymal cells in culture with wheat germ agglutinin-gold conjugate and grafted (injected) them orthotopically into the anterior, middle or posterior regions of the presomitic mesoderm of mouse embryos at the four to seven somite stage. Labelled cells colonized somites that formed from the region into which they were injected and also from more posterior regions. The study concluded that there is a general posterior movement of cells in the presomitic mesoderm." I do not agree that these experiments demonstrate that mixing of cells normally occurs among somitomeres. Somitomeres in the segmental plate (or the presomitic mesoderm) are in the process of becoming epithelialized so their cell-surface characteristics likely differ one somitomere to another. Culturing of cells is known to change cell surface characteristics, so the cells labelled in culture and injected probably contained a mix of surface characteristics and sorted out accordingly. Even if some cells do mix among the somitomeres, the same is true of many other structures, for example, Scott Fraser showed that about 5% of the cells of neuromeres mix with adjacent neuromeres. That does not mean that neuromeres do not exist. "The contention that metameric patterns must have compartment properties is a gratuitous assumption. Morphology is often ahead of final patterning in embryos. We must remember that vertebrate embryos may regulate even rather late in development, such as the well-known case of the eye-forebrain field of a salamander persisting as late as the optic vesicle stage so an explanted optic vesiicle will still form an eye and a forebrain. Somitomeres are metameric morphological units that form somites even if they pass around a few cells. "Some people claim that cranial somitomeres do not exist. For example, Jacob et al. (37) reported a computer assisted study of densities of nuclei (thus cell densities) in somites and cranial somitomeres. They concluded "that a metameric pattern does not exist rostral to the first somite" (their Page 85). Since all somitomeres begin as expansion figures, and the pre- and subotic somitomeres continue to expand along with the brain rather than condense as somites do, this study is a non sequitur, based on a false premise. Furthermore, 1 and 2 are quite clearly shown in the scanning electron micrograph of their Fig. 7, the legend for which says "A metemeric pattern is not recognisable in the paraxial mesoderm". A similar, though smewhat more extensive, study was published in 1996 by Freund et al. (38), entitled "The metameric pattern of the head: does it exist?" They conclude it does not, but their study is based on the same false premise as the one just mentioned, and thus is another non sequitur." "Some published criticisms of somitomeres are rather brief. For example, cranial somitomeres are dismissed in half a sentence by Lumsden and Keynes (39). They say "In contrast to the trunk, paraxial mesoderm segmentation in the head is inconsequential, ...". An unsupported opinion like that is of no consequence." The relationships between somitomeres and neuromeres is illustrated above and reviewed several times (eg. (20)). Cranial somitomeres most likely influence the segmentation of the hindbrain and the positions of the cranial nerves. 1. B.H. Lipton and A.G. Jacobson (1974) Analysis of Normal Somite Development. Devel. Biol. 38:73-90. 2. B.H. Lipton and A.G. Jacobson (1974) Experimental Analysis of the Mechanisms of Somite Morphogenesis. Devel. Biol. 38:91-103. 3. D.S. Packard, Jr., and A.G. Jacobson (1976) The Influence of Axial Structures on Chick Somite Formation. Devel. Biol. 53:36-48. 4. S. Meier (1979) Development of the Chick Embryo Mesoblast: Formation of the Embryonic Axis and Establishment of the Metameric Pattern. Devel. Biol. 73:25-45. 5. S. Meier (1981) Development of the Chick Embryo Mesoblast: Morphogenesis of the Prechordal Plate and the Cranial Segments. Devel. Biol. 83:49-61. 6. A.G. Jacobson (1998) Somitomeres. From "Principles of Medical Biology", Volume 11, Developmental Biology, Chapter 11, pages 209-228. JAI Press Inc., Stamford, CT. 7. D.S. Packard, Jr. and S. Meier (1983) An Experimental Study of the Somitomeric Organization of the Avian Segmantal plate. Devel. Biol. 97:191-202. 8. S. Meier (1982) The Development of Segmentation in the Cranial Region of Vertebrate Embryos. Scanning Electron Microscopy/1982, pt 3:1269-1282. 9. S. Meier (1984) Somite Formation and its Relationship to Metameric Patterning of the Mesoderm. Cell Differentiation 14:235-243. 10. R.L.Triplett and S. Meier (1982) Morphological Analysis of the Development of the Primary Organizer in Avian Embryos. J. Exp. Zool. 220:191-206. 11. P.P.L. Tam and S. Meier (1982) The Establishment of a Somitomeric Pattern in the Mesoderm of the Gastrulating Mouse Embryo. Am. J. Anatomy 164: 209-225. 12. S. Meier and P.P.L. Tam (1982) Metameric Pattern Development in the Embryonic Axis of the Mouse. l. Differentiation of the Cranial Segments. Differentiation 21: 95-108. 13. P.P.L. Tam, S. Meier, and A.G. Jacobson (1982) Differentiation of the Metameric Pattern in the Embryonic Axis of the Mouse. II. Somitomeric Organization of the Presomitic Mesoderm. Differentiation 21: 109-122. 14. A.G. Jacobson (1993) Somitomeres: Mesodermal Segments of the Head and Trunk. Chapter 2 in: The Skull. Volume 1. Development. Pages 42-76. J. Hanken and B.K. Hall, Eds. University of Chicago Press, Chicago and London. 15. D.S. Packard,Jr. and S. Meier (1984) Morphological and Experimental Studies of the Somitomeric Organization of the Segmental Plate in Snapping Turtle Embryos. J. Embryology and Experimental Morphology 84:35-48. 16. S. Meier and D.S. Packard, Jr. (1984) Morphogenesis of the Cranial Segments and Distribution of Neural Crest in the Embryos of the Snapping Turtle, Chelydra serpentine. Devel. Biol. 102: 309-323. 17. A.G. Jacobson (2001) Somites and Head Mesoderm Arise from Somitomeres. In: The Origin and Fate of Somites. E.J. Sanders, J.W. Lash, and C.P. Ordahl, Eds. IOS Press, Amsterdam. 2001. pp.16-29. A pdf of this paper is here . 18. A.G. Jacobson and S. Meier (1984) Morphogenesis of the Head of a Newt: Mesodermal Segments, Neuromeres, and Distribution of Neural Crest. Devel. Biol. 106: 181-193. 19. E.S. Goodrich (1930) Studies on the Structure and Development of Vertebrates. Macmillan, London (republished by Dover, New York, 1958). 20. A.G. Jacobson and S. Meier (1986) Somitomeres: The Primordial Body Segments. In: Somites in Developing Embryos. R. Bellairs, D.A. Ede, and J.W. Lash. Plenum Publishing Corp, 1986, pp.1-16. 21. M.Q. Martindale, S.Meier, and A.G. Jacobson (1987) Mesodermal Metamerism in the teleost: Oryzias latipes (the Medaka). Journal of Morphilogy 193:241-252. 22. H.L. Hamilton (1952) Lillie's Development of the Chick. An Introduction to Embryology. pp147-148. Holt, New York. 23. D.M. Noden (1983) The Embryonic Origins of Avian Cephalic and Cervical Muscles and Associated Connective Tissues. American J. of Anatomy 168: 257-276. 24. D.M. Noden (1983) The Role of the Neural Crest in Patterning of Avian Cranial, Skeletal, Connective, and Muscle Tissues. Devel. Biol. 96: 144-165. 25. G. Couly, P.M.Coltey, and N.M. Le Douarin (1992) The Developmental Fate of the Cephalic Mesoderm in Quail-Chick Chimeras. Development 114: 1-15. 26. M.A. England (1986) Aspects of Somite Formation in the Early Chick Embryo. In: R. Bellairs, D.A. Ede, and J.W. Lash (eds.), Somites in Developing Embryos, NATO ASI Series, Vol. 118, Plenum Press, New York and London, pp. 147-160. 27. O. Cleaver and P.A. Krieg (1998) VEGF Mediates Angioblast Migration During Development of the Dorsal Aorta in Xenopus. Development 125: 3905-3914. 28. C.M. Cox and T.J. Poole (2000) Angioblast Differentiation is Influenced by the Local Environment: FGF-2 Induces Angioblasts and Patterns Vessel Formation in the Quail Embryo. Devel. Dynamics 218: 371-382. 29. H. Damas (1944) Recherches sur le Développement de Lampetra fluviatilis L. Contribution à l'étude de la Cephalogenèse des Vertébrés. Archs. Biol. Paris 55: 1-289. 30. D.M. Noden (1988) Interactions and fates of Avian Craniofacial Mesenchyme. Development 103: 121-140. 31. C.D. Stern and R.J. Keynes. Cell Lineage and the Formation and maintenance of Half Somites. In: R. Bellairs, D.A. de and J.W. Lash (eds.), Somites in Developing Embryos, NATO ASI Series, Vol. 118, Plenum Press, New York and London, (1986) pp. 1147-1590. 32. A.G. Jacobson. (1994) Normal Neurulation in Amphibia. In: Neural Tube Defects (CIBA Foundation Symposium (81), Wiley, Chichester, pp/ 6-24. 33. B. Menkes and C. Miclea. (1962) Cercetari Asupra Formii Organclor Axial. III. Posibilitatile de Vindecare si Reorganizare a Mezodermului Axial Presomitic in Urma Disocierii Mecanice. Formarea de Somite Supra-numerare, Stud. Cerc. St. Med. (Timisoara) 9: 203-228. 34. B. Menkes and S. Sandor. (1969) Researches on the Formation of Axial Organs in the Chick Embryo. V., Rev. Roum. Embryol. Cytol.-Serie Embryol. 6: 65-72. 35. S. Sandor. (1972) Researches on the Formation of Axial Organs in the Chick Enbryo. VIII. Some New Aspects of Regulation Potencies During Somitogenesis. Rev. Roum. 8: 113-121. 36. P.P.L. Tam. (1988) The Allocation of Cells in the Presomitic Mesoderm During Somite Segmentation in the Mouse Embryo. Development 103: 379-390. 37. M. Jacob et al., On the Problem of Metameris the Head Mesenchyme of Chick Embryos. In: R. Bellairs, D.A. Ede and J.W. Lash (eds.) Somites in Developing Embryos, NATO ASI Series, Vol. 118. Plenum Press, New York and London, (1986) pp. 79-89. 38. R. Freund et al. (1996), The Metameric Pattern of the Head: Does It Exist? Anat. Embryol. 193: 73-80. 39. A. Lumsden and R. Keynes (1989) Segmental Patterns of Neuronal Development in the Chick Hindbrain. Nature 337: 424-428. |