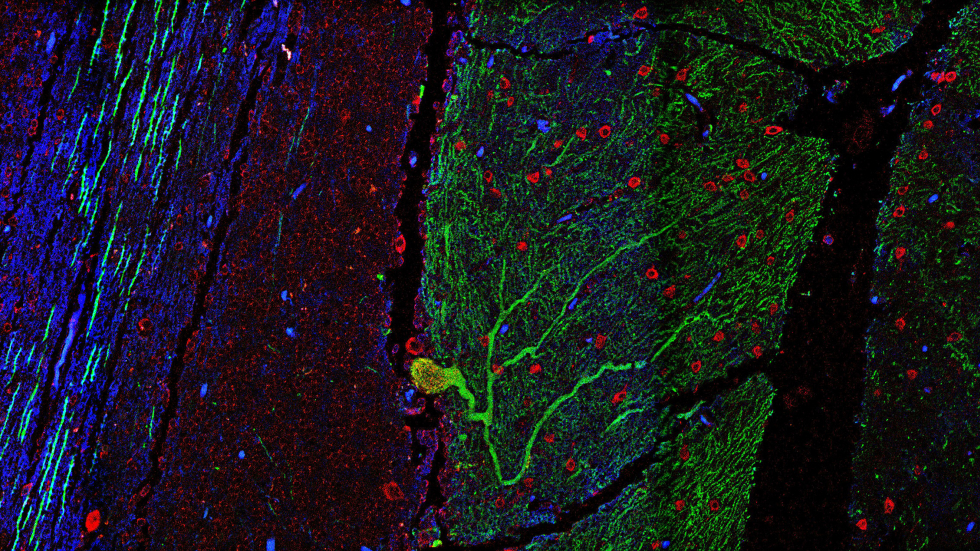
. “Characterization of DNA-PK-Bound End Fragments Using GLASS-ChIP.” Methods Mol Biol, 2444, Pp. 171-182. Abstract
Welcome to the Paull Lab
Research in the Paull lab is focused on the DNA damage response in eukaryotic cells, specifically the checkpoint activation and DNA repair responses that occur immediately after the introduction of chromosomal double-strand breaks. Several components of these DNA damage response systems have been implicated as tumor suppressors in mammals, and nearly all of the proteins we study are involved in the maintenance of genomic stability in eukaryotic organisms. We are also interested in the regulation of redox control and signaling that occurs in response to oxidative stress in human cells. To study these processes, we use biochemistry and molecular biology-based tools to understand how critical proteins in these pathways function and are regulated in response to stress. Learn more...