|
The experiments described in some detail below are illustrated graphically in a following section: "Graphic Illustrations of Nose, Lens and Ear Induction". A link to this section is here. Lens epidermis and eye relationships in the newt (from 17). 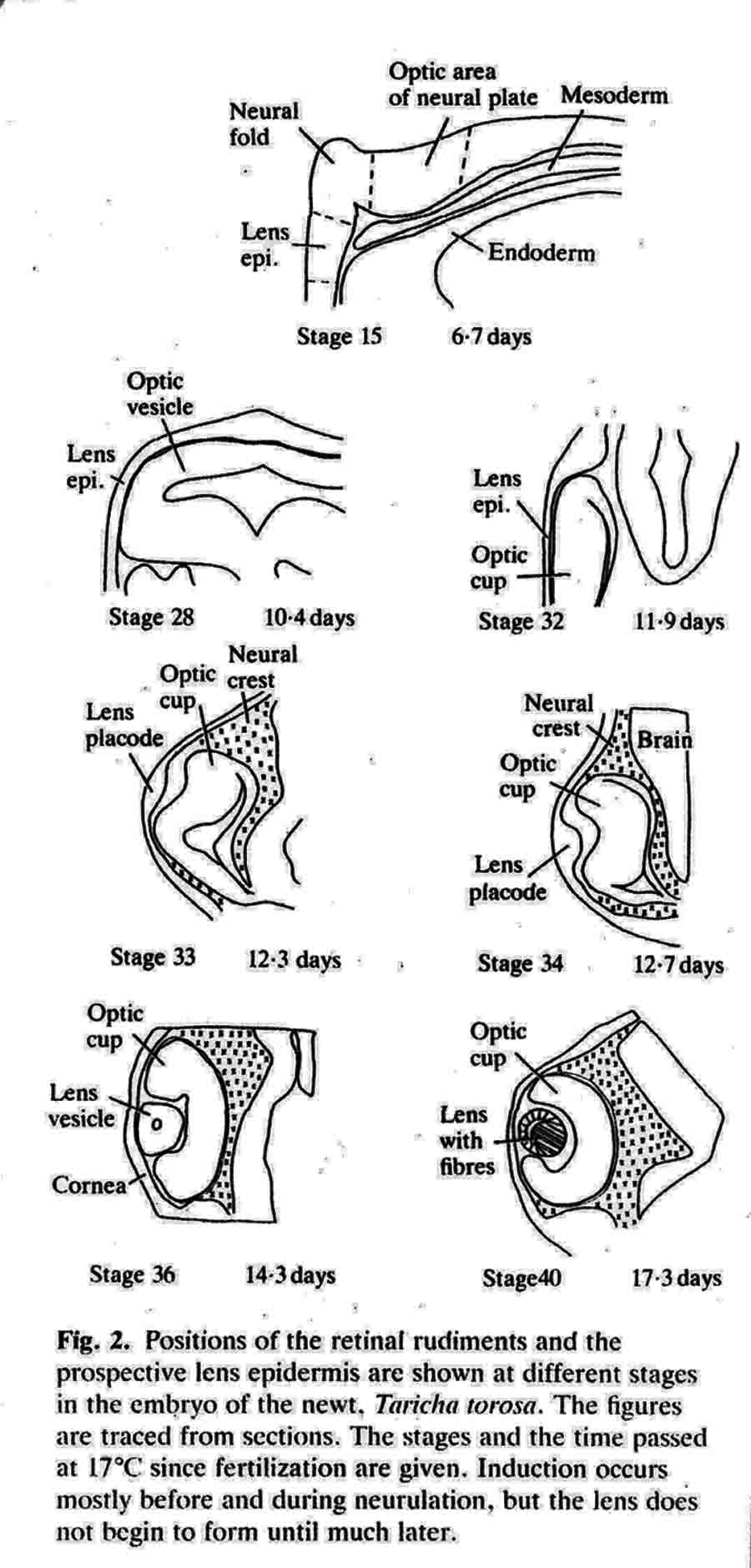 Liedke, (3,4,5) in other amphibian species, found that interactions with embryonic tissues other than the optic vesicle contributed to lens "competence". In 1951, fifty years after Spemann did his work and shortly after Liedke did her work, I took as my doctoral research (at Prof. Twitty's suggestion) the combination on a single species, the West Coast newt, Taricha torosa , of temperature studies and the seeking of other possible inductors of the lens besides the optic vesicle. By means of defect and explant experiments, I established that tissues that underlay, directly or nearby, the prospective lens ectoderm during gastrula and neurula stages were inductors of lenses. These tissues are the anterior dorsolateral head endoderm and the prospective heart mesoderm. These two inductor tissues act together, or can act alone separately. These inductor tissues can evoke lenses when putative induction from the prospective retina is excluded. Endoderm begins the induction process before the mesoderm is encountered. My experiments indicated little or no role of the retina in lens elicitation. 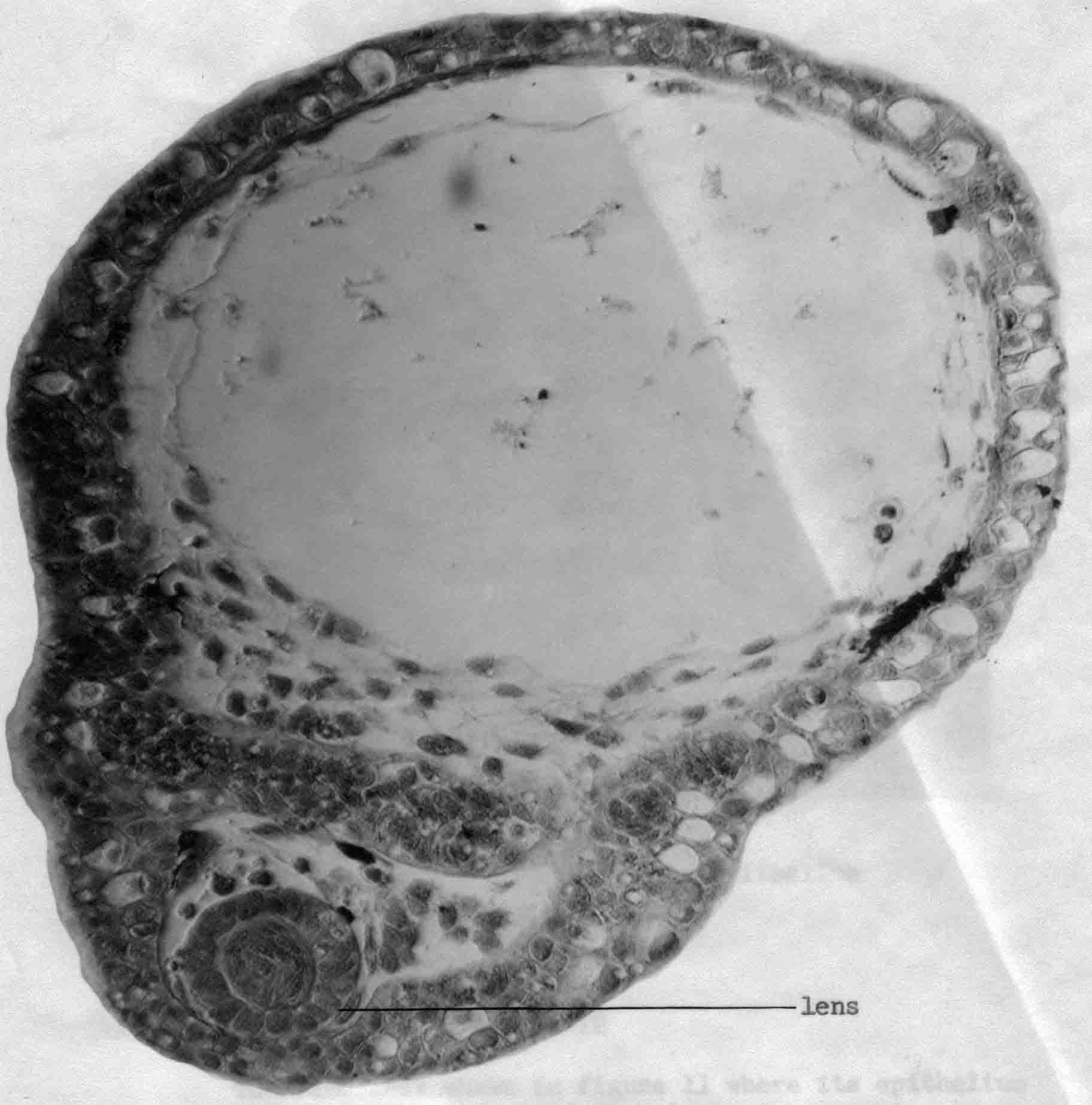 A large lens with extensive fiber formation induced in an embryo from which the entire anterior neural plate, which includes the prospective eyes, was removed. I established that the temperature limits that early embryos of Taricha could survive were 5 degrees C and 25 degrees C. About half the embryos reared at either of these two temperatures survived and developed (these are the species LT/50's). The rate of development is greatly affected by temperature; for example, the time needed to go from cleavage stages to mid-neurula stage was 3 days at 25 degrees C and 25 days at 5 degrees C. The optimum temperature for development (and lens production) was 16-17 degrees C. Different optima are found for different species. Twitty (6) had earlier found that low temperatures slowed the rate of determination more than the rate of morphological development. One may conclude that embryos reared at low temperatures are retarded in the induction process. My experiments were published in 1955 (7) and 1958 (8). As a lens emerges from the ectoderm, it begins as a thickening or placode. Other so-called placodal structures of the head include those of the nasal epithelia and the placodes that become the inner ears (otic placodes). I expanded my studies to include the induction pattern of these placodes, and to ask the question "How is the rather precise location of these placodal structures determined?" Experiments that explanted the strip of ectoderm from which the structures will emerge, with and without putative inductors, as well as experiments in which the strip of ectoderm was rotated 180 degrees and re-implanted, helped reveal the sequence of inductor tissues for each of the organs, and curiously how these induction abilities overlapped among the organ positions (9,10,11). Depending upon the timing of the operations, one can coax ears out of the nose regions, and noses out of the ear regions. I have concluded that the normal positions of nose, lens and ear are fixed by the summed intensity of the inductors experienced. These nose, lens, and ear experiments were presented diagrammatically in a Science article in 1966 (12). This article summarizes my early views of induction. A pdf of this paper is here . The evidence indicates that induction is a long cumulative process including separable positive and inhibitory interations. Induction is not a trigger event, and lens induction takes at least two weeks at 17 degrees Celsius in the newt embryo. Effects of several different inductor tissues are additive, and all seem to be doing the same thing, or at least substiture for one another. What has been called "competence" is likely the result of induction occurring in early development. A fuller understanding of lens determination has emerged since the experiments described above were done. It is now clear that some tissues will induce an organ, and other tissues may inhibit or suppress emergence of the organ. Von Woellwarth (13), in 1961, demonstrated that neural crest cells inhibit lens development, and that the optic vesicle's close association with the prospective lens ectoderm prevented neural crest cells from interacting with the lens area. Later (1987 and 1988) experiments by Henry and Grainger (14) and Grainger, Henry and Henderson (15) confirmed the inhibitory effects of neural crest on lens development. I re-examined my own data and found that they also suggested inhibition of lens induction by the neural crest cells. Besides the lens, neural crest cells inhibit induction of several other organs. I reviewed some of this topic in 1992 (16). A review of embryonic induction with Amy Sater (17) (pdf here ) adds considerably to the story of lens induction through 1988. We list 37 species of fishes, amphibia, birds and mammals that have been tested for the ability to form lenses in the absence of the optic vesicle. Twenty-five of the 37 species formed lenses in the absence of the retina. The underlying endoderm and the heart mesoderm establish a lens field. Later, the optic vesicle presses against the overlying prospective lens ectoderm and excludes the inhibitory migrating cranial neural crest cells from direct contact with the part of the lens field from which the lens emerges. The placodes that form the nasal epithelium and the inner ear are induced in similar ways as the induction of the lens. To quote from the Jacobson and Sater review (17): "The nose is induced primarily by underlying anterior endoderm and to some extent by the forebrain. The ear is induced by its underlying mesoderm and the nearby hindbrain. Experiments on the head ectoderm that forms nose, lens and ear (9,10,11) led to the following conclusions. (A) The endodermal and/or the mesodermal inductors have the dominant role in inducing and positioning nose, lens and ear. The neural structures that contribute to their induction have only a secondary importance. Each placodal structure can form in the absence of induction from the neural structures. (B) The separate capabilities to induce nose, lens and ear are spread in gradient fashion in the endoderm and mesoderm. That part of the head epidermis that sees the highest amount of induction for an organ forms that organ (see figure). What the responding tissue 'sees' is complicated by the morphogenetic movements occurring in both inductors and responding tissue. (C) The head epidermis is simultaneously responding to the different inductors for nose, lens and ear (and probably other organs as well). When the responding tissue reaches the response threshold necessary to form one of the organs, that organ will form and other organs are excluded. These results demonstrate that a responding tissue may at the same time be at some level of commitment for several organs. This concept has not been generally appreciated, however, it has significance for the interpretation of molecular events and hypotheses for induction" 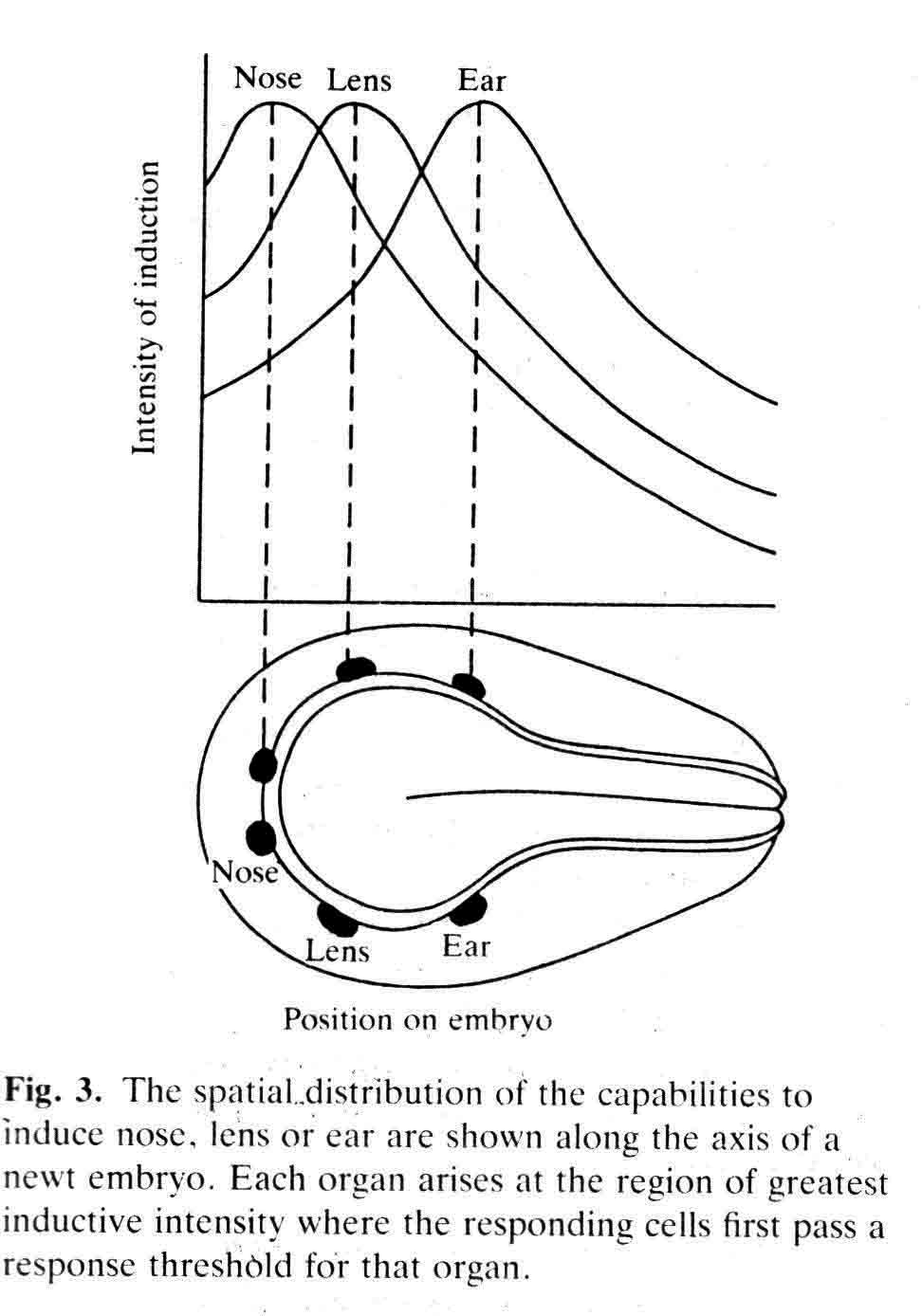 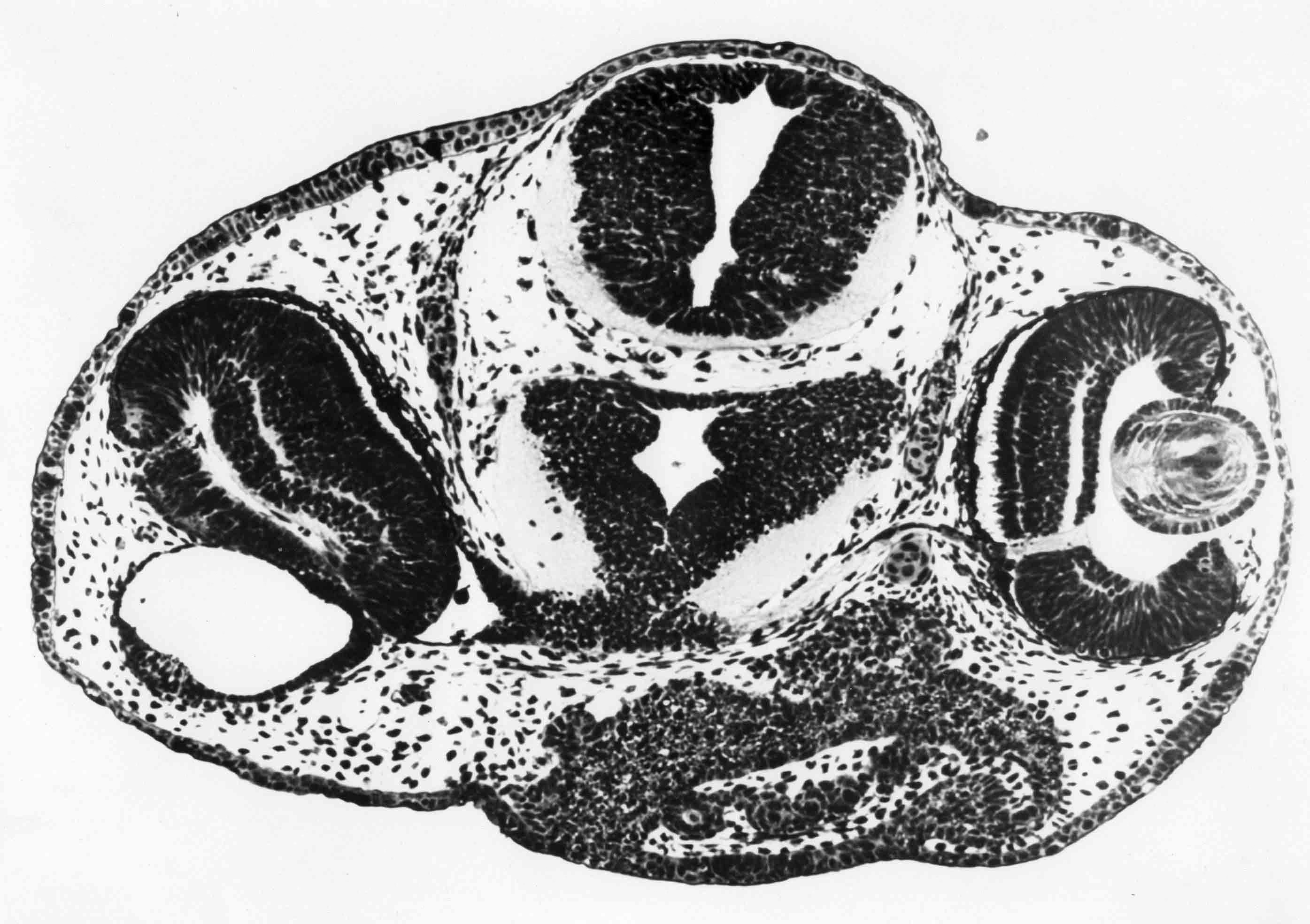 Finally, the graph below (from 17), shows how the non-linear read-out from percent reponses of experiments comes about. 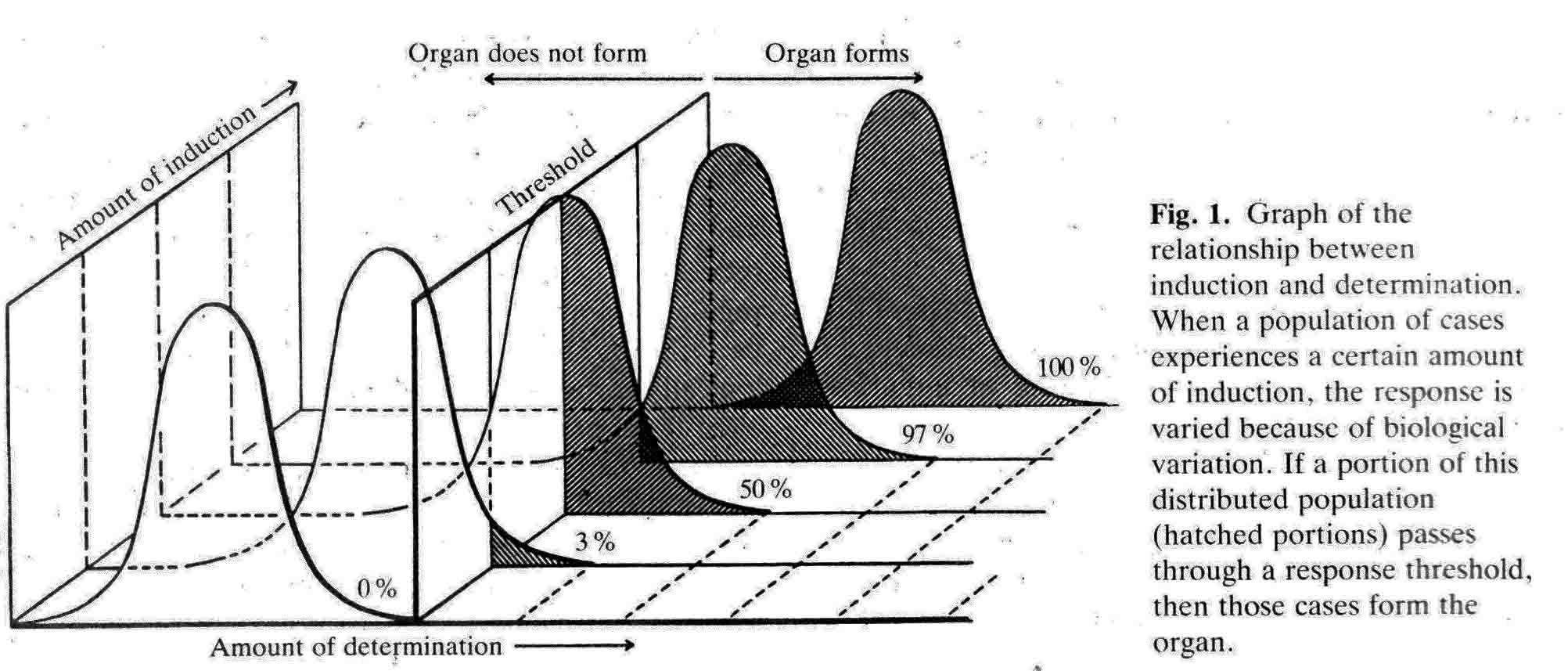 (1) 1901 H. Spemann. Uber Korrelationen in der Entwicklung des Auges.Verh, Anat, Ges. 15 Vers. Bonn 61-79. (2) G. Ten Cate 1953 The Intrinsic Development of Amphibian Embryos. North Holland Publishing Company, Amsterdam [Research during the war years.] (3) K.B. Liedke 1942 Lens competence in Rana pipiens. J. Exp. Zool., 90:331-351. (4) __________ 1951 Lens competence in Amblystoma punctatum. J. Exp. Zool., 117:573-591. (5) __________ 1953 Studies on lens induction in Amblystoma punctatum. J. Exp. Zool. 130:353-373. (6) V.C. Twitty 1928 Experimental Studies on the ciliary action of amphibian embryos. J. Exp. Zool. 50:319-344. (7) 1955 A.G. Jacobson. The roles of optic vesicle and other head tissues in lens induction. Proc. Nat. Acad. Sci. USA 41:522-525. (8) 1958 A.G. Jacobson. The roles of neural and non-neural tissues in lens induction. J. Exp. Zool. 139:525-557. (9) 1963a A.G. Jacobson. The determination and positioning of the nose, lens and ear. I. Interaction within the ectoderm and between the ectoderm and underlying tissues. J. Exp. Zool. 154:273-284. A pdf is here . (10) 1963b A.G. Jacobson. The determination and positioning of the nose, lens and ear. II. The role of the endoderm. J. Exp. Zool. 154:285-292. A pdf is here . (11) 1963c A.G. Jacobson. The determination and positioning of the nose, lens and ear. III. Effects of reversing the antero-posterior axis of epidermis, neural plate, and neural folds. J. Exp. Zool. 154:293-304. A pdf is here . (12) 1966 A.G. Jacobson. Inductive processes in embryonic development. Science 152:25-34. A pdf is here . (13) 1961 C. Von Woellwarth. Die Rolle des neuralleistenmaterials und der Temperatur bei der Determination der Eugenlinse. Embryologia 6:219-242. (14) 1987 J. J. Henry & R.M. Grainger. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev. Biol. 124:200-214. (15) 1988 R.M. Grainger, J.J. Henry & R.A. Henderson. Reinvestigation of the role of the optic vesicle in embryonic lens induction. Development 102:517-526. (16) 1992 A.G. Jacobson. Induction and pattern emergence in the mesoderm. In: Formation and Differentiation of Early Embryonic Mesoderm. Edited by Bellairs, et al., Plenum Press, New York. (17) 1988 A.G. Jacobson and A.K. Sater. Features of embryonic induction. Development 104:341-359. |