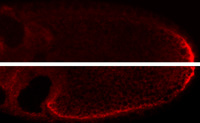
Localized (top) vs mislocalized (bottom) osk mRNA
Strategies in localization of mRNAs
Localization of mRNAs is mediated by cis-acting localization signals. For mRNAs with complex programs of localization there can be several different signals, each interacting with different proteins. A poorly understood aspect of mRNA localization is how the mRNA is transferred between different localization machineries or between localization and anchoring components. We recently characterized the various signals that direct the first step in localization of osk mRNA, in which the mRNA is transported from the nurse cells to the oocyte. Although the signals act together to direct very efficient transport, they are individually weak. We proposed that weak association of individual signals with the transport machinery was necessary to allow transfer to the separate machinery that then delivers the mRNA to the posterior pole of the oocyte. Replacing a weak osk transport signal with a strong signal disrupted posterior localization (image at right), supporting this model.
We are now extending this project, using a combination of genetic, biochemical and imaging approaches. Positions in the lab are open now (September 2017) to contribute to this project. Please contact Paul Macdonald if you are interested.
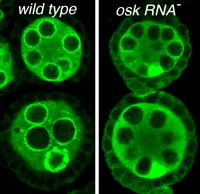
Bruno spreading to follicle cells
and depleted from nuage when osk RNA is absent
Noncoding RNA function required for progression through oogenesis
The osk gene is best known for its role in directing assembly of germ plasm at the posterior pole of the Drosophila oocyte, a process that initiates posterior patterning of the embryo and formation of the embryonic primordial germ cells. This function requires the Osk protein. Curiously, the osk RNA also has a noncoding function that is separate from the protein function and required earlier during oogenesis. In the absence of osk RNA oogenesis is arrested. We recently identified specific defects of the osk RNA null mutants, including effects on nuage organization or assembly and a relaxation of the distinction between germ line and somatic cells in the ovary. We also mapped the sequences contributing to osk ncRNA activity, identifying two general types of essential elements. One class of element includes the signals that direct transport of osk to the oocyte: loss or substantial reduction of transport dramatically disrupts osk ncNRA activity. The second class of element appears to act most directly, and includes a cluster of binding sites for several proteins.
We are now extending this project, using a combination of genetic, biochemical and imaging approaches. Positions in the lab are open now (September 2017) to contribute to this project. Please contact Paul Macdonald if you are interested.